The deep mycoses are uncommon in our setting. These fungal infections occur mainly in immunosuppressed patients or in tropical climates, and include subcutaneous infections and systemic infections. The skin is always involved in the former. In the first part of this review, we describe the main subcutaneous mycoses: sporotrichosis, chromoblastomycosis, mycetoma, phaeohyphomycosis, hyalohyphomycosis, and lacaziosis. Early recognition and treatment is important, as these infections are frequently associated with high morbidity.
Las micosis profundas son infecciones poco frecuentes en nuestro medio. Se presentan principalmente en pacientes inmunodeprimidos o en regiones de climas tropicales, que abarcan las micosis subcutáneas y las micosis sistémicas. Las micosis subcutáneas o por implantación siempre producen signos de afectación cutánea. En la primera parte de esta revisión se realizará una revisión de las principales micosis subcutáneas: esporotricosis, cromoblastomicosis, micetomas, feohifomicosis, hialohifomicosis y lacaziosis. Reconocer y tratar estas micosis subcutáneas de forma precoz es importante, ya que a menudo están asociadas a una alta morbilidad.
The deep mycoses are uncommon infections caused by fungi; they are divided into subcutaneous and systemic mycoses.1 While skin manifestations always occur in subcutaneous mycoses, or mycoses of implantation, as they are also known, they are only occasionally seen in systemic mycoses. In such cases, the skin is affected either directly, by the penetration of the fungus into the dermis, or indirectly, by an infection that has spread from a deeper focus. According to Rezusta et al.,2 the majority of subcutaneous and systemic mycoses in Spain are imported, with just a few exceptions (e.g., mucormycosis). Epidemiological data on the prevalence and incidence of mycoses in Spain are lacking.
Subcutaneous MycosesThe subcutaneous mycoses comprise several clinical entities caused by invasion of the skin and subcutaneous tissue by saprophytic fungi that live in soil and vegetation. However, even though cuts and wounds are very common in people living in rural areas, overall, there are very few cases of subcutaneous mycoses.1
The typical route of entry for the fungus is traumatic inoculation through contaminated material such as splinters, thorns, or other sharp objects, explaining why subcutaneous mycoses are also referred to as mycoses of implantation.3
Although the fungi responsible for subcutaneous mycoses are taxonomically heterogeneous, they are unified by the fact that they share the same route of entry. Any of these infections can affect people who have traveled to endemic areas, even years after their return.
The most common subcutaneous mycoses are sporotrichosis, chromoblastomycosis, and mycetoma.1 Other less common entities are lacaziosis, phaeohyphomycosis, hyalohyphomycosis, and conidiobolomycosis.
SporotrichosisSporotrichosis is a subacute or chronic infection caused by dimorphic fungi, the most common of which is Sporothrix schenckii.4,5 These fungi are universal, although they are more common in tropical and subtropical areas. The estimated incidence of sporotrichosis in South America is between 48 and 60 cases per 100000 population a year.6,7 Only a few autochthonous cases have been reported in Spain and other parts of Europe,8 and the majority of cases in these areas are imported.9
The causative agents belong to a species complex known as S schenckii,10,11 which comprises Sporothrix brasiliensis, Sporothrix mexicana, Sporothrix luriei, Sporothrix pallida (previously Sporothrix albicans), and Sporothrix schenckii sensu lato (sl.), which is the most common of the five.12
After an incubation period of 15 to 30 days, traumatic inoculation by Sporothrix spp. results in a chronic infection characterized by nodular lesions in the cutaneous and subcutaneous tissue associated with lymphangitis in the affected area.
Sporothrix spp. live in vegetation, plants, or plant debris in the soil, and therefore infections are more common in agricultural workers and people working in open areas. Sporotrichosis is considered an occupational disease in forest wardens, horticulturists, gardeners, and farm workers in general.4,13 Alcoholism and diabetes have also been described as risk factors. Immunosuppression, regardless of the cause, is also a predisposing factor for disseminated or systemic disease.1 The disease can also be acquired through manipulation of the fungus in a laboratory setting. Finally, there was an interesting epidemic in southern Brazil in which sporotrichosis was transmitted to humans through cat scratches, suggesting that it might be a zoonotic infection.14 Most of the species isolated in these cases were S brasiliensis.
Clinical Forms5- 1)
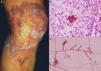
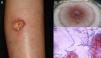
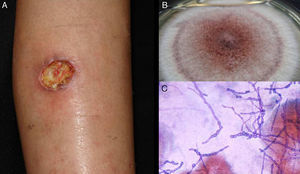
Lymphocutaneous sporotrichosis. Also known as lymphangitic sporotrichosis, this clinical form accounts for over 75% of all cases of sporotrichosis.15 The lesions occur in exposed areas, such as the hands, face, and feet. The disease starts as a painless purple or blackish nodule that erodes into a small ulcer (sporotrichotic chancre) with swollen edges, a painful granulomatous center, and minimal discharge. This is followed by lymphangitis with secondary nodules along the line of lymphatic drainage that can progress to ulcers; this characteristic pattern is known as sporotrichoid spread (Fig. 1).6 The patient's general health is not affected.15 The course of disease varies according to the host's immune response, the virulence of the strain, the size of the inoculum, and the depth of the lesion.
- 2)
Fixed sporotrichosis. This variant is characterized by the presence of a solitary lesion. The infection is limited and generally presents as a slow-growing, less progressive verrucous plaque. Fixed sporotrichosis does not normally affect the lymph vessels and is more common in endemic areas.16
- 3)
Other clinical forms:
Osteoarticular sporotrichosis. This is a disseminated form of sporotrichosis that affects the bones and joints; it is the most common form of systemic involvement.17
Primary pulmonary sporotrichosis. This variant preferentially affects immunosuppressed patients and is acquired by inhalation. It mimics cavitary tuberculosis.16
Metastatic pulmonary sporotrichosis. The metastatic form of pulmonary sporotrichosis is uncommon and has only been described in isolated cases. It occurs in immunocompromised patients, particularly those with human immunodeficiency virus (HIV) infection in the AIDS stage.18
Widespread invasion. Disseminated disease is rare in sporotrichosis, although meningeal and ocular involvement have been described in immunosuppressed patients with uncontrolled diabetes or chronic alcoholism.
In Mexico, like other countries in Latin America (home to the largest case series and the most experience with sporotrichosis), lymphocutaneous sporotrichosis accounts for 60% to 80% of all cases of sporotrichosis, fixed cutaneous sporotrichosis for 10% to 30%, and other clinical forms for 1% to 2%.16
Sporotrichosis must be distinguished from tuberculosis, leishmania, tularemia, cutaneous nocardiosis, nontuberculous mycobacterial infections, mycetoma, chromoblastomycosis, and lepromatous leprosy. Sporotrichoid (lymphangitic) spread can be seen in many of these conditions, which must be contemplated in the differential diagnosis.19
Diagnosis- 1.
Pus (aspirated from nodules). Direct microscopic examination is of no value in sporotrichosis, as lesions contain very few yeast forms. Sabouraud dextrose agar (SDA) and SDA with antibiotics (chloramphenicol and cycloheximide) can be used for culture, which produces yeast colonies that are initially white and then darken (Fig. 1). Growth is characteristically quick (3-5 days)20 but 2 weeks are needed to identify the fungus and confirm diagnosis.16 Molecular identification by polymerase chain reaction (PCR) analysis is also possible.10,21
- 2.
Histology. Histologic examination reveals a nonspecific mixed granulomatous reaction with neutrophilic microabscesses. The fungus presents as a small cigar-shaped yeast form sometimes surrounded by characteristic radiating eosinophilic material known as an asteroid body. While asteroid bodies can aid diagnosis, they are not pathognomic,16,20 as they are also found intracellularly in sarcoidosis, silicosis, and lacaziosis (lobomycosis). Extracellular asteroid bodies, however, are more characteristic of sporotrichosis. Several specimens may be needed to visualize the microorganisms, although they are easier to find in the case of disseminated or visceral disease.
Sporotrichosis may resolve spontaneously in some cases, such as during pregnancy, although paradoxically dissemination has also been reported in pregnant women.
- 1.
Saturated solution of potassium iodide. Treatment with potassium iodide, as a saturated solution, is started at 5 drops per meal. This initial dose is then gradually increased to 20 or 30 drops per meal according to tolerance levels. The treatment should be maintained for 3 to 4 weeks after resolution of the clinical manifestations. The mechanism of action is unknown, although potassium iodide is thought to act as an immunostimulant. Adverse effects include a metallic taste in the mouth, rhinitis, expectoration, urticaria, petechiae, bullous or acneiform rash, vasculitis, and induction of hypothyroidism or hyperthyroidism. Potassium iodide is contraindicated during pregnancy.20,22
- 2.
Itraconazole 200mg/d for 3 to 6 months.22 This is the first-line treatment recommended in most treatment guidelines. It tends to be a little more expensive than potassium iodide, but it has fewer adverse effects.
- 3.
Other options. Terbinafine 250-1000mg/d for 3 to 6 months23,24; fluconazole 400mg/d for 3 to 6 months22; amphotericin B (deoxycholate) 0.5-1mg/kg/d for systemic disease or liposomal or lipid formulations of amphotericin B at a dose of 3-5mg/kg/d22; local heat or thermotherapy for 2 or 3 months,22 or a combination of the above treatments (potassium iodide with itraconazole, itraconazole with terbinafine, and terbinafine with potassium iodide).25 The addition of photodynamic therapy with methyl aminolevulinate or even better intralesional methylene blue 1% (combined or not with itraconazole) has produced good results in vitro and in 1 patient.26
Surgery can have an important role in osteoarticular sporotrichosis.22 Debridement and arthrodesis were traditionally considered the treatments of choice but prosthetic joint replacement followed by long-term antifungal treatment has also been described as a viable option
1.1Chromoblastomycosis (Chromomycosis)Chromoblastomycosis, also known as chromomycosis, is a chronic polymorphic fungal infection of the skin and subcutaneous tissue. It is caused by several species of melanized or dematiaceous fungi, which produce a dark pigment. The parasitic forms of these fungi are called fumagoid or muriform cells (sclerotic bodies).27–30
The most common species that cause chromoblastomycosis are Fonsecaea pedrosoi, Fonsecaea monophora, Cladophialophora carrionii, Phialophora verrucosa, and Rhinocladiella aquaspersa.5,27,28 Most patients have a history of a traumatic injury involving wood or vegetation, and over 80% are rural workers in Africa, Asia, and South America who tend to walk barefoot. The fungi responsible for chromoblastomycosis have been found worldwide, though they are more common in tropical and subtropical countries.27
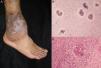
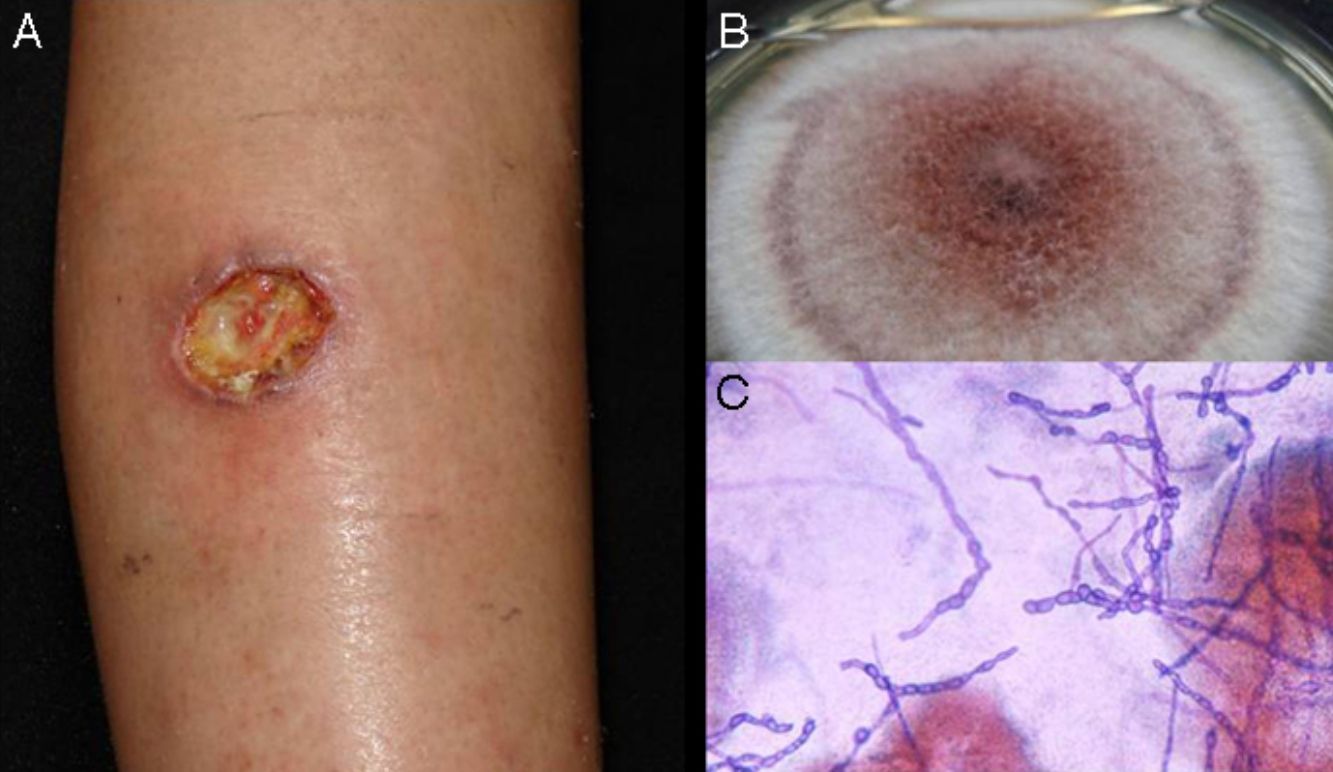
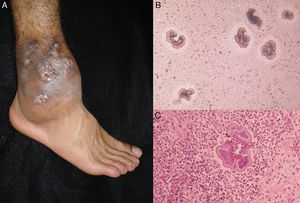
Clinical FormsThe fungus generally penetrates the skin through a skin injury, typically located on the lower limbs.31 About 1 or 2 months later, the infected individual develops a papule that progresses to a slow-growing warty nodule (Fig. 2). The infection is limited to the subcutaneous tissue and does not spread to either muscle or bone, except in immunocompromised patients. Individual lesions can develop a thick cauliflower-like appearance and bacterial superinfection is common. Secondary lymphedema, possibly progressing to elephantiasis, and squamous cell carcinoma may occur.27
Diagnosis- 1.
Direct examination. Direct examination of crusts and fragments of skin can reveal parasitic forms that occur in isolation or form characteristic septa (Fig. 2). The microscopic structures observed are common to all species.27,28
- 2.
Culture. The fungi that cause chromoblastomycosis grow slowly when cultivated on SDA with or without antibiotics (chloramphenicol and cycloheximide); they produce dark olivaceous or black colonies with a flat velvety surface and a raised center. Distinction between species is difficult and is based on reproductive structures and molecular identification.30 Molecular biology techniques (PCR), in particular targeting internal transcribed spacer (ITS) regions of ribosomal DNA (rDNA), are also useful.32,33
- 3.
Histology. Histologic examination shows characteristic pseudoepitheliomatous hyperplasia in the epidermis and a mixed granulomatous inflammatory infiltrate with giant cells containing characteristic round fungal structures (Fig. 2) in the dermis.27
Chromoblastomycosis is extremely difficult to treat and is often refractory to diverse options, including nonpharmacological treatments such as curettage, electrocoagulation, and cryosurgery.5 Antifungals must be maintained for at least 6 months, and while they may produce a favorable clinical outcome, recurrences during or after therapy are common. Treatment should be terminated when all the lesions disappear.27
Other treatments include surgical resection of small lesions; local cryosurgery (in association with an antifungal to prevent lymphatic spread); itraconazole 200-400mg/d alone or combined with 5-fluorocitosine 30mg/kg 4 times a day for 6 months; terbinafine 250-500mg/d for 12 months, and in the case of systemic involvement intravenous amphotericin B at a dose of 1mg/kg or liposomal or lipid formulations of amphotericin B at a dose of 3-5mg/kg/d.27
MycetomaMycetoma is a chronic local infection caused by several species of fungi and bacteria. The infection is called actinomycetoma when it is caused by aerobic filamentous bacteria and eumycetoma when it is caused by fungi.34 It is characterized by the formation of aggregates of the causative microorganisms in abscesses. These aggregates are known as grains or granules. Granules can drain through sinuses opening onto the skin or affect adjacent bones. The disease advances via direct spread, with very few cases of dissemination to distant sites. The causative agents are generally found in the soil and they enter the body through broken skin. Most cases involve rural workers.
Etiology- 1.
Fungi. The fungi that cause eumycetoma produce white or dark granules. They are particularly common in Africa, India, and Mexico. Dark granules are formed by Madurella mycetomatis, Trematosphaeria grisea, and Leptosphaeria senegalensis,35 while white granules are formed by Fusarium spp., Acremonium spp., and Aspergillus nidulans.
- 2.
Filamentous bacteria or aerobic actinomycetes. The granules formed by these species are red (Actinomadura pelletieri), white-yellow (Actinomadura madurae, Nocardia brasiliensis, and Nocardia spp.), or yellow-brown (Streptomyces somaliensis). Actinomycetes are found all over the world, not just in tropical countries.36
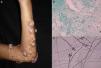
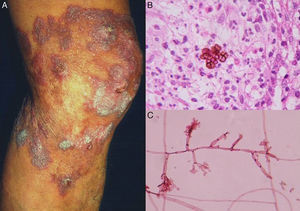
The clinical characteristics of mycetoma caused by fungi and actinomycetes are very similar. Lesions are more common on the feet, shins, and hands. The earliest clinical manifestation is a hard painless nodule that spreads slowly to produce papules and sinuses that discharge fluid containing granules onto the skin surface.35,36 The original site of infection is distorted by local tissue swelling, formation of chronic sinuses, and late bone involvement (Fig. 3). Lesions are rarely painful, except in late stages.
DiagnosisMycetoma granules (Fig. 3) are a key diagnostic finding and are generally found on examining discharge from sinuses or on crushing a crust taken from a lesion. Microscopic examination will show whether these granules are formed by small actinomycetes or wider mycotic filaments. Definitive identification requires culture, which is normally carried out on SDA with or without antibiotics (chloramphenicol and cycloheximide); chloramphenicol alone is preferred in the case of hyaline fungi. The agents can also be identified by molecular biology testing, particularly PCR analysis using different markers37 depending on the causative agents (e.g., ITS regions of rDNA, β-tubulin,38 and D1/D2). Partial ribosomal RNA gene sequence analysis, by contrast, can be used to identify Nocardia and Actinomadura species.36 Histologic findings are similar in all forms of mycetoma, and include an inflammatory center rich in polymorphonuclear cells (true abscesses), epithelioid cells, giant cells, and fibrosis. The granules are located in the center of the inflammation.35,39 Imaging studies, while complementary, can aid diagnosis by showing soft tissue swelling, osteolytic lesions, and cortical thickening.
The differential diagnosis should include bacterial osteomyelitis, tuberculous osteomyelitis, hidradenitis suppurativa, Kaposi sarcoma, and cutaneous tuberculosis, among others.35,39
TreatmentActinomycetoma. The treatment regimen with the strongest evidence base for nocardial mycetoma is trimethoprim-sulfamethoxazole plus diaminodiphenyl sulfone (dapsone) for 6 months to 2 years. Amoxicillin-clavulanic acid, administered over 6 months, can be used for refractory cases.40–42 The treatment of choice for extensive infection and/or visceral involvement is amikacin combined with trimetoprim-sulfametoxazol39 or meropenem.43,44 There have been isolated reports of successful outcomes with other agents in patients who do not respond to these treatments.36,39,45
Eumycetoma. Unlike in actinomycetoma, where pharmacological treatment is associated with good outcomes, the standard treatment in eumycetoma is a combination of medical treatment and surgery. Acceptable results have been reported for the use of last-generation triazoles, such as itraconazole and fluconazole used alone or in combination with terbinafine. These drugs are administered over a long period and only after exhausting all surgical options.35,45
PhaeohyphomycosisPhaeohyphomycosis is a heterogeneous group of mycoses caused by dark-walled (dematiaceous) fungi.46,47 These fungi are found in all climates, although they are more common in tropical climates. There has been a recent rise in cases among immunosuppressed patients with HIV infection or AIDS, transplant recipients, and diabetic patients, among others.46,48
The most common causative agents are Exophiala spp., Bipolaris spp., Curvularia spp., Pleurophomopsis spp., Phaeoacremonium spp, and Alternaria spp. The fungi are found mainly in organic debris.
Clinical Forms- 1.
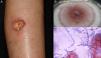
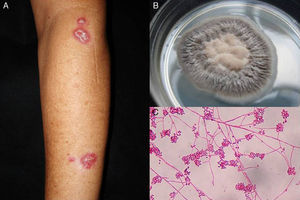
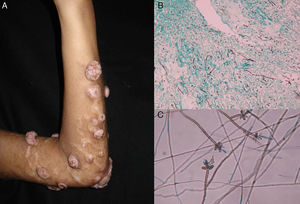
Subcutaneous phaeohyphomycosis. Following local trauma or inoculation with foreign material, patients develop a slow-growing solitary lesion (generally a cyst or a nodule, or possibly a plaque or abscess) normally located on the extremities (Fig. 4).48,49 The differential diagnosis should include lipomas, epidermal or synoviale cysts, fibromas, foreign body cysts, and bacterial abscesses.
- 2.
Systemic or disseminated phaeohyphomycosis. While very rare, systemic phaeohyphomycosis is very serious in immunosuppressed patients.50
Wet-mount microscopy shows diagnostic dark septate hyphae forming branches or chains (Fig. 4). Growth is slow (3-4 weeks) on SDA and colonies acquire an olivaceous or dark brown color. PCR analysis of markers such as β-tubulin and ITS regions can be used for molecular identification.51,52 Biopsy reveals a cyst wall formed by palisading macrophages with mycotic hyphae.49
TreatmentTreatment of infections caused by Exophiala spp. is controversial, and one option that has been proposed is surgical resection.48 There are also no standard protocols for the treatment of Alternaria infections.53 The best option for phaeohyphomycosis appears to be a combination of antifungal therapy (itraconazole, ketoconazole, or terbinafine) and surgery. Exophiala spp. strains tend to be resistant to fluconazole. Disseminated infections are treated with amphotericin B.48,49
HyalohyphomycosisHyalohyphomycosis is caused by hyaline fungi (Hyphomycetes) that form septate hyphae in tissue.47 This classification, however, is rather arbitrary as there are many types of terrestrial and aquatic Hyphomycetes. Just a few organisms, however, can cause infections, most of which are opportunistic, in humans.54,55 Most of the genera involved in hyalohyphomycosis are morphologically identical when observed in tissue sections and they trigger the same pathologic response. Fungi that frequently cause infections or have another particularly distinctive characteristic are assigned to a different category (e.g., aspergillosis).
The most common agents involved in hyalohyphomycosis are Aspergillus (fumigatus, niger, flavus), Scopulariopsis spp., Fusarium spp., Acremonium recifei, Paecilomyces spp., Purpureocillum spp., and Neoscytalidium spp.55 They are all widely distributed in nature, and can be found in any type of soil, wood, or decomposing plant material.56 They affect individuals of either sex and at any age, and immunosuppression is not a necessary condition for infection.
Clinical FormsHyalohyphomycosis can be classified as superficial, subcutaneous, or systemic.
- 1.
Superficial hyalohyphomycosis. Superficial infections include dermatomycosis and onychomycosis. They are common in rural workers, fishermen, patients with severe burns, and premature neonates.57,58
- 2.
Subcutaneous hyalohyphomycosis. Traumatic inoculation causes abscesses, cysts, and tumor-like lesions similar to those seen in mycetoma (Fig. 5).56
- 3.
Systemic hyalohyphomycosis. Systemic infections, while uncommon, are very serious. They affect immunosuppressed patients and can be fatal. Hematogenous and lymphatic spread leads to involvement of the lungs and central nervous system.55,57
Identification of septate hyaline hyphae by microscopic examination of skin scales, nail fragments, secretions, or fragments provides a presumptive diagnosis, which is then confirmed by culture (Fig. 5). Most fungi grow on SDA without antibiotics or inhibitors.47,59 As in the cases described above, molecular identification is also possible.60
The differential diagnosis should include other dermatomycoses, epidermal cysts, actinomycetoma, eumycetoma, histoplasmosis, and cryptococcosis.
TreatmentIn immunocompetent individuals, the treatments of choice are triazoles, terbinafine, or surgery.61 When the immune system is compromised, the first-line treatment is amphotericin B combined with a triazole (itraconazole 200mg/d for 6 months or fluconazole 150mg twice a week for 6 months).
Lacaziosis (Lobomycosis)Lacaziosis, which was formerly known as lobomycosis, is a chronic granulomatous fungal infection of the skin and subcutaneous tissues first described under the name of keloidal blastomycosis in 1930 by Jorge Lobo in Recife, Brazil.62 It is a rare infection found in Central and South America; it is caused by Lacazia loboi,62,63 a yeast that cannot be grown in culture. The source of infection is thought to be in soil and vegetation. The fungus probably enters through the skin following a penetrating injury, such as a thorn prick or insect bite.
Lacaziosis is characterized by keloidal lesions with well-defined lobulated edges in exposed areas of the body (frequently the face, arms, or legs). The lesions spread to contiguous sites, although transmission to distant sites is also possible via autoinoculation.
DiagnosisDiagnosis is facilitated by the identification of abundant fungal structures during direct examination and chains of diffuse round cells connected by small tubular structures in biopsy samples.62 Causative agents can also be identified in tissue by PCR analysis, in particular assays targeting the 18S rDNA fragment.64
The differential diagnosis should include keloids, lepromatous leprosy, and anergic leishmania.
TreatmentAntifungals are not effective in lacaziosis and the definitive treatment is surgical resection.62,63
ZygomycosisZygomycosis is a heterogeneous group of fungal infections caused by opportunistic Zygomycetes of the orders Mucorales (Rhizopus, Lichtheimia, Mucor, and Rhizomucor) and Entomophthorales (Basidiobolus and Conidiobolus).65 In this section, we will only discuss Entomophthorales fungi, as the Mucorales are addressed in the second part of this review, which looks at systemic mycoses.
Entomophthoromycosis is characterized by the appearance of a hard, progressive mass that affects the subcutaneous tissues. There are 2 variants. The first is caused by Basidiobolus ranarum and is more common in children.66 Lesions generally appear in the shoulder and pelvic girdles, and present as a slowly spreading woody cellulitis. The second variant is caused by Conidiobolus coronatus and affects adults. The primary infection starts in the lower turbinates of the nose and then spreads to the center of the face, causing painful indurated swelling and severe deformation of the nose, lips, and cheeks.65–68
ConclusionWe have reviewed the main characteristics of the subcutaneous mycoses and the main diagnostic and treatment methods available (Table 1).
Summary of the Characteristics of the Subcutaneous Mycoses.
| Mycosis | Main Causative Agent | Diagnosis | Treatment |
|---|---|---|---|
| Sporotrichosis | Sporothrix schenckii | Cultivation of pus aspirated from nodules; histology; PCR | Saturated solution of potassium iodide for 3 wk; itraconazole 200mg/d for 3–6 mo; combinations |
| Chromoblastomycosis | Fonsecaea pedrosoi | Microscopic examination; culture; histology | Very difficult. Itraconazole 200mg/d for 6 mo; terbinafine for 12 mo; amphotericin B; combinations |
| Mycetoma | Filamentous actinomycetes and filamentous fungi | Identification of mycetoma granules; culture; histology; PCR | Antibiotics for actinomycetoma; antifungals+surgery for eumycetoma |
| Phaeohyphomycosis | Exophiala jeanselmei Alternaria spp. | Microscopic examination and culture; histology; PCR | Controversial. Surgery+combination of antifungals |
| Hyalohyphomycosis | Opportunistic Aspergillus (fumigatus, niger, flavus), Fusarium spp., Paecilomyces, etc. | Microscopic examination and culture; PCR | Immunocompetent patients: triazoles; terbinafine; ciclopirox olamine; surgery Immunosuppressed patients: amphotericin B combined with a triazole for 6 mo |
| Lacaziosis (lobomycosis) | Lacazia loboi | Microscopic examination; histology; PCR | Surgery |
| Entomophthoromycosis | Conidiobolus coronatus Basidiobolus ranarum | Direct examination and culture | Surgery and systemic antifungals |
Abbreviation: PCR, polymerase chain reaction.
The authors declare that they have no conflicts of interest.
Please cite this article as: Carrasco-Zuber JE, Navarrete-Dechent C, Bonifaz A, Fich F, Vial-Letelier V, Berroeta-Mauriziano D. Afectación cutánea en las micosis profundas: una revisión de la literatura. Parte 1: micosis subcutáneas. Actas Dermosifiliogr. 2016;107:806–815.