Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease. Treatment for moderate-to-severe HS includes biologic therapies (adalimumab and secukinumab), resulting in increased disease management costs. Our aim was to estimate the difference between secukinumab and adalimumab in terms of pharmacological costs and costs per responding patient 1 year into therapy from the Spanish National Health System (NHS) perspective.
Material and methodsWe designed a decision tree comparing different treatment sequences, starting with a different first-line therapy. Patients switched arms based on achieving HS clinical response ≥50% (based on the SUNSHINE, SUNRISE, and PIONEER clinical trials results). A cohort of 100 patients was considered. Only treatment costs in € (2023 base year) were considered for the analysis. A panel of experts validated the model structure and parameters.
ResultsAfter 52-weeks into therapy, treatment sequences in the secukinumab group resulted in a total cost of €1,198,912, corresponding to €16,858 per responder. Total costs in the adalimumab treatment group were 2.5% higher, corresponding to €19,701 per responder. A total of 80% of responders who start treatment with secukinumab do not change treatment, while only 31% of responders who start treatment with adalimumab stay on the same treatment.
ConclusionsThe results of our financial assessment can help decision makers in selecting the most efficient therapeutic approach for treating patients with moderate-to-severe HS and poses secukinumab as a suitable therapeutic option for the Spanish NHS.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease characterized by the inflammation of hair follicles. It typically emerges after puberty and manifests as painful lesions, affecting the axilla, inguinal, perianal, and gluteal regions.1,2 In Spain, according to different estimations the prevalences is estimated to be around 0.5%.3
Treatment for HS aims to control the inflammation and reduce pain through changes in lifestyle and medical and surgical therapies.4 Traditional therapies includes topical keratolytics, antiseptics, and antibiotics, alongside systemic treatment involving antibiotics, retinoids, and corticosteroids.5,6 Biological therapies have been incorporated into the therapeutic arsenal, being of special relevance for patients with moderate-to-severe HS.5,7 Up to 2023 adalimumab was the only biologic treatment approved for HS treatment based on the results of the PIONEER studies.8,9 However, the efficacy profile of adalimumab in the routine clinical practice is variable and primary or secondary lack of efficacy may occur.7 In February 2023 the European Medicines Agency (EMA) approved secukinumab, a monoclonal antibody that binds to IL-17A, as a therapeutic alternative. Secukinumab has already been approved for psoriasis, psoriatic arthritis, and axial spondylarthritis.10 The SUNSHINE and SUNRISE trials, conducted with patients with HS, demonstrated that a higher proportion of patients on secukinumab achieved a clinical response in HS clinical response (HiSCR50) vs patients on placebo 52-weeks into therapy.11 Based on this, secukinumab was approved for the treatment of HS.10
Former studies have shown that the use of biological therapies results in an increased cost of the treatment for HS.12,13 In this regard, financial assessments are essential to provide patients with HS with the best therapeutic approach available. To date, no economic evaluations on the use of secukinumab for HS have been published.
Considering the above, the main goal of the study was to estimate the difference between secukinumab and adalimumab regarding the pharmacological cost and the cost per responding patient one year after treatment initiation from the perspective of the Spanish National Health System (NHS).
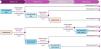
Materials and methodsModelWe designed a decision tree considering secukinumab and adalimumab therapies during a 52-week regimen (Fig. 1). The model allows to compare different treatment sequences. Each treatment sequence starts with a different first-line therapy (secukinumab or adalimumab). Efficacy is evaluated at weeks 16 and 52 for the secukinumab group and at weeks 12 and 36 for the adalimumab one based on results from the SUNSHINE and SUNRISE,11 and PIONEER8 clinical trials for secukinumab and adalimumab, respectively. Patients achieving a HiSCR of ≥50% (50% reduction in total abscess and inflammatory nodule count, with no increase in abscess count, and no increase in draining fistula count relative to baseline14) were categorized as responders and stayed in their current treatment group. If treatment fails, a switch in treatment occurs.
Patients from the secukinumab group switch to secukinumab boost (secukinumab q2w) at week 16, as per label.15 Non-responders from the secukinumab q2w group further switch to adalimumab at week 32 (after 16 weeks on secukinumab q2w) to keep treating patients and explore a new molecule with a different mechanism of action.
Patients from the adalimumab group with no clinical response at either week 16 or 36 (weeks of efficacy assessment in the adalimumab clinical trial) switch to secukinumab, since the label does not consider up-titration.16 Those failing to respond at week 32 switch to secukinumab q2w (Fig. 1).
The model structure and parameters used have been validated by a panel of 3 experts (2 dermatologists and 1 hospital pharmacist) with extensive expertise in the management and treatment of HS.
PopulationThe hypothetical cohort included in the model included a total of 100 adults with moderate-to-severe HS who were eligible to receive a biological agent.
TreatmentsThe model included the two biological therapies currently approved for the treatment of moderate-to-severe HS that were reimbursable in Spain up to March 2023: secukinumab 300mg, administered initially at weeks 0, 1, 2, 3 and 4 and then monthly during the maintenance phase,15,17 and adalimumab administered with an initial dose of 160mg on day 1 followed by 80mg on days 15 and 29, continuing the maintenance phase with a dose of 40mg administered weekly.16,17
Treatment efficacyClinical response rates, assessed using the HiSCR50, were obtained from a pooled analysis of the results of the SUNSHINE and SUNRISE trials for secukinumab,11 and the PIONEER trials for adalimumab8 (Fig. 1).
The SUNSHINE and SUNRISE trials evaluated secukinumab q4w and secukinumab q2w efficacy at weeks 16 (percentage of patients with clinical response: 47.0% and 48.6%, respectively) and 52 (percentage of patients with clinical response: 78.6% and 79.8%, respectively).11 Response rates to treatments in our decision tree is measured at different timeframes. Accordingly, in our model, the 16-week trial response rates were applied to patients treated for less than 30 weeks, whereas the 52-week response rates were applied to patients treated for more than 30 weeks.
On the other hand, the PIONEER trials evaluated adalimumab efficacy HiSCR50 at week 12 and 36.8 We calculated the average from the results obtained at these weeks for PIONER I and PIONER II, determining that 50.4% of patients showed good responses at week 12 and 48.8% at week 36. In our model, adalimumab response rate at week 16 was assumed to be the same as in week 12. In the absence of data at week 52, we conservatively assumed equal efficacy for adalimumab and secukinumab.
Additionally, the rates of secukinumab rescue and secukinumab q2w rescue (secukinumab given after failure to respond to adalimumab) used were assumed to be the same as for first-line secukinumab and secukinumab q2w as the response was similar in the clinical trials.11
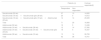
CostsOnly treatment costs (€, 2023) were considered for the analysis. Prices for secukinumab were obtained from Spanish sources.17–19 The use of secukinumab was applied according to the confidential special conditions of price and reimbursement agreed between the company and the Spanish Ministry of Health in February 2024. Price for adalimumab was calculated using the mean acquisition price and market share for adalimumab and biosimilars in Spain (2020–2022),20 which is 22.0% lower than the list price19 and an additional discount of 20.0% is applied. The number of doses of each treatment, across all treatment sequences is shown in Table 1.
Cost per treatment sequence.
| Treatment sequence | Secukinumab doses(n) | Secukinumab doses Q2(n) | Adalimumab doses(n) |
|---|---|---|---|
| Secukinumab (52 wk) | 15 | 0 | 0 |
| Secukinumab (16 wk)→Secukinumab q2w (36 wk) | 7 | 16 | 0 |
| Secukinumab (16 wk)→Secukinumab q2w (16 wk)→Adalimumab (20 wk) | 7 | 7 | 22 |
| Adalimumab (52 wk) | 0 | 0 | 54 |
| Adalimumab (36 wk)→Secukinumab (16 wk) | 7 | 0 | 38 |
| Adalimumab (16 wk)→Secukinumab (36 wk) | 12 | 0 | 18 |
| Adalimumab (16 wk)→Secukinumab (16 wk)→Secukinumab q2w (20 wk) | 7 | 9 | 18 |
q2w: twice a month; wk: weeks.
Differences of pharmacological treatment costs and costs per responder at week 52 were calculated. The number of responders was the sum of patients achieving HiSCR50 at week 52.
Primary failure cost was calculated for each treatment by counting the number of non-responder patients and the cost of doses at week 16 and following evaluation periods. For the adalimumab treatment group, the cost of secukinumab induction phase was considered as well.
Alternative scenariosAn alternative scenario was developed to assess the robustness of cost analyses for each treatment sequence. The scenario considers an acquisition cost discount of 45.0% for adalimumab, the average discount for biosimilars.
ResultsBase case scenarioTo ease the interpretation of model results, a total of 100 patients with HS were considered for each treatment group. After the 52-week regimen, all treatment sequences in the secukinumab treatment group resulted in a total cost of €1,198,912, which corresponds to €16,858 per responder (n=71). The total costs in the adalimumab treatment group were 2.5% higher than the secukinumab treatment group, corresponding to €19,701 per responder (n=62) (Table 2). Overall, the cost per responder in the treatment sequences initiated with secukinumab resulted in a difference of €2843 per responder in favor of secukinumab, 14.4% lower than those on adalimumab (Table 2).
Cost per treatment sequence, treatment, and responders after the 52-week regimen.
| Patients (n) | Cost per responder(€) | ||
|---|---|---|---|
| Responders | Non-responders | ||
| Secukinumab (52 wk) | 37 | 10 | 14,835 |
| Secukinumab (16 wk)→Secukinumab q2w (36 wk) | 20 | 5 | 14,612 |
| Secukinumab (16 wk)→Secukinumab q2w (16 wk)→Adalimumab (20 wk) | 14 | 14 | 25,529 |
| Total | 71 | 29 | 16,858 |
| Adalimumab (52 wk) | 19 | 5 | 13,438 |
| Adalimumab (16 wk)→Secukinumab (36 wk) | 12 | 14 | 27,392 |
| Adalimumab (16 wk)→Secukinumab (16 wk)→Secukinumab q2w (20 wk) | 18 | 5 | 16,347 |
| Adalimumab (36 wk)→Secukinumab (16 wk) | 13 | 14 | 26,769 |
| Total | 62 | 38 | 19,701 |
q2w: twice a month; wk: weeks.
Based on the efficacy data considered, in the modelling after 1 year into therapy, 80.3% (57 out of 71) of responders who start treatment with secukinumab do not change treatment, while only 30.6% (19 out of 62) of responders who start treatment with adalimumab remain on the same therapy (Table 2).
The cost of secukinumab doses represented almost 90% of the total cost in the treatment sequences initiated with secukinumab, while for the treatment sequences initiated with adalimumab, the cost of adalimumab doses represented a 40.8% (Fig. 2).
During the induction phase, 53 patients from the secukinumab group were considered to be non-responders. This resulted in costs associated with primary failure of €288,393, all of which were attributable to the administration of secukinumab. On the other hand, 50 patients did not respond to adalimumab the first 16 weeks into therapy, resulting in a total primary failure cost of €367,782. Of these, €174,808 corresponded to adalimumab treatment, and €192,975 to secukinumab induction.
Alternative scenarioFor the alternative scenario, the results showed the same trend as in the base case (Table 3) with a cost per responder for secukinumab treatment sequences of €16,337 (€16,566 for adalimumab).
Cost per treatment sequence and treatment. Alternative scenario.
| Cost per responder(€) | |
|---|---|
| Secukinumab (52 wk) | 14,835 |
| Secukinumab (16 wk)→Secukinumab q2w (36 wk) | 14,612 |
| Secukinumab (16 wk)→Secukinumab q2w (16 wk)→Adalimumab (20 wk) | 22,858 |
| Total | 16,337 |
| Adalimumab (52 wk) | 9,239 |
| Adalimumab (16 wk)→Secukinumab (36 wk) | 22,450 |
| Adalimumab (16 wk)→Secukinumab (16 wk)→Secukinumab q2w (20 wk) | 14,947 |
| Adalimumab (36 wk)→Secukinumab (16 wk) | 24,476 |
| Total | 16,566 |
Overall, the cost per responder for the treatment sequences initiated with secukinumab showed that the costs were very similar, with a difference of €230 per responder in favor of secukinumab.
DiscussionIn this study, we conducted a pharmaco-economic evaluation of the secukinumab use for the treatment of moderate-to-severe HS vs the use of adalimumab from the perspective of the Spanish NHS, using a cost-consequence analysis.
The analysis showed that, based on previously published efficacy rates, initiating treatment for hidradenitis suppurativa with secukinumab resulted in a higher number of responders compared with initiation of adalimumab. Notably, treatment with secukinumab could be exclusive, without the need for dose escalation or switching, whereas treatment with adalimumab may require rescue therapy with secukinumab. In addition, initiation with secukinumab was associated with lower total pharmacologic costs and lower cost per responder compared with initiation with adalimumab. However, when a 45.0% discount was applied to the cost of adalimumab, a lower cost per responder was observed.
Our results are consistent with those from previously published studies in other conditions such as psoriasis21 and psoriatic arthritis22 in which secukinumab demonstrated to be the most efficient treatment from the Spanish NHS perspective vs other biological agents. This was attributed to its efficacy profile and persistence of the effect, resulting in the greatest number of responders with a cost containment, which translates into the lowest cost per responder. Of note, for HS management, the standard dose for adalimumab is administered every week, which doubles the dose and cost vs other diseases as psoriasis, psoriatic arthritis, or axial spondylopathies.
The efficacy data for both treatments were obtained from previous clinical trials. However, clinical trials for adalimumab did not consider the 52-week timepoint and for this, clinical response rates were assumed to be the same as for secukinumab. In this context, the model developed for this study was conservative for secukinumab, as real-life studies have reported clinical responses rates for adalimumab at 52 weeks from 53.9%23 to 72.1%,24 which is lower than that assumed for this study (78.6%).
The management of HS not only inflicts a significant financial strain on patients but also affects their work productivity.25,26 Former European studies have indicated that the predominant contributors to HS-related costs are treatment expenses, time off work due to sickness and decline in work productivity.12,13 Given the higher number of responders with secukinumab compared with adalimumab, both direct costs related to hospital-based patient management and indirect costs, including those associated with reduced work productivity, may be decreased.
Similarly, HS has a significant negative impact on patients’ HRQoL, including high levels of pain, anxiety, and depression, being higher in those with moderate-to-severe HS.27 In this regard, a recently published study showed that, compared with placebo, secukinumab improved patients’ HRQoL.28 This, along with the results of our study showing that the early use of secukinumab results in a higher number of responders, suggests that using secukinumab as a first-line therapy could help achieve a better disease control and ultimately reduce the disease burden.
For the management of HS, the concept of “window of opportunity” was proposed and defined as that period in the disease course in which efforts to control the disease are more effective and patients can obtain the best results.29 In this regard, the results of our study show that starting HS therpy with secukinumab would be beneficial, as a higher proportion of patients in this treatment group were considered responders and resulted in lower treatment costs vs patients treated with adalimumab.
Our study has some limitations. First, due to the lack of long-term efficacy data for adalimumab in clinical trials, we assumed the clinical response rates from SUNRISE and SUNSHINE clinical trials. Second, the model assumes that patients who do not achieve a response discontinue treatment, because partial responses could not be incorporated owing to a lack of data. Third, in the absence of data on patients who fail biologic therapy and subsequently receive rescue treatment with another biologic or treatment intensification, efficacy estimates from the corresponding clinical trials in biologic-naïve patients were assumed. Further research is warranted to evaluate treatment sequencing when local comparative studies in similar populations become available.
Fourth, for the model in our study, we assumed timepoints for efficacy evaluation slightly different from those in the clinical trials to compare both treatments. Fifth, inclusion criteria for clinical trials assessing the efficacy secukinumab and adalimumab were not the same thus, this could have influenced clinical response rates. Sixth, discontinuation rates were not considered in the decision tree due to the short-term follow-up of our analysis, as they should have minimal impact. Finally, due to the nature of the model used, only the cost of pharmacological treatment is considered, although the total cost of patient management would include other costs such as surgery. However, it has been shown that there is a correlation between good pharmacological control and the need for fewer surgical interventions when patients are treated with secukinumab. Despite the above-mentioned limitations, we tried to address them in the most conservative approach not overestimating the efficacy and persistence data for secukinumab and giving adalimumab its best possible results published or estimated.
Secukinumab has been approved in Spain as second-line therapy after failure of adalimumab. This analysis suggests that, when considering that adalimumab is not providing enough efficacy for the patients, there are no economical reason for delaying the switch to secukinumab. The results of our economic evaluation can help decision makers in selecting the most efficient therapeutic approach for treating patients with moderate-to-severe HS and poses secukinumab as a suitable therapeutic option for the Spanish NHS.
CRediT authorship contribution statementAll authors of the manuscript (AMC, AML, AJM and CB) have contributed to the conception and design of the model and the interpretation of the data; they have participated in the critical review of the intellectual content of the manuscript and have approved its final version prior to publication.
Conflict of interestThe authors declare no conflict of interest.
We thank Outcomes’10 (a ProductLife Group Company) for their contributions and support throughout the study, both in concept, design and medical writing.