While the prevalence of congenital syphilis continues to be low throughout most of the developed world, there has been a slight resurgence of the disease in several European countries, including Spain. In this context, we need to become more familiar with the signs and symptoms of this disease and consider its diagnosis in patients with only mild clinical manifestations. A definitive diagnosis may be difficult or even impossible in patients whose diagnostic tests reveal low positive titers or inconsistent results. The cornerstone of congenital syphilis control is prenatal screening and the treatment of infected mothers with penicillin, an effective and economical intervention. Based on a review of the literature supplemented by data from our own clinical experience, this article provides a detailed description of the clinical manifestations of congenital syphilis as well as the various diagnostic methods and treatments available.
La sífilis congénita, aunque poco frecuente en la mayoría de los países desarrollados, ha experimentado un ligero resurgimiento en varios países de Europa, entre los que cabe incluir a España. Necesitamos, por lo tanto, familiarizarnos más con sus síntomas y signos, y reconsiderar el diagnóstico de sífilis congénita en pacientes con síntomas poco manifiestos. Además, en aquellos pacientes que presentan en los test diagnósticos títulos bajos o inconsistentes, el diagnóstico de sífilis congénita puede ser difícil o incluso imposible. La piedra angular del control de la sífilis congénita es la detección prenatal en madres y el tratamiento con penicilina, intervención eficaz y rentable. En este artículo se describen con detalle, a partir de los datos aportados por la literatura, las manifestaciones clínicas de la sífilis congénita, así como las distintas formas de su diagnóstico y terapéutica. Asimismo, el presente trabajo se enriquece con datos procedentes de nuestra propia experiencia clínica.
Congenital syphilis—a form of the disease which has been recognized for over 500 years—is usually caused by transmission of the spirochete Treponema pallidum subspecies pallidum across the placenta, but infection may also occur during birth.
Excellent historical medical treatises have described the devastating early and late clinical and pathologic manifestations of congenital syphilis, and modern publications have documented the less aggressive clinical presentations of the disease following the discovery of penicillin.
Pre-Columbian congenital syphilis has been postulated based on the discovery of apparently syphilitic bone lesions in skeletons from Neolithic burial sites, and skeletons with characteristic signs of this disease were also found in Roman Pompeii.
Syphilis was so common in the 16th century that the humanist Erasmus of Rotterdam (1469-1536) noted with some cynicism that a man would hardly be taken for a nobleman without the French pox, indeed would not be thought much of a man. Both the Austrian and Spanish branches of the Hapsburgs suffered greatly from the disease, and Philip II of Spain, his son Charles, and his third wife, Elisabeth of Valois, all had symptoms of the congenital form. Philip IV contracted syphilis and infected his second wife, Mariana of Austria, who transmitted the disease to their son, who would reign as Charles II. There was also syphilis among the Austrian Hapsburgs: examples are Franz Joseph I and his wife Elisabeth (known as Sissy), as well as their son Rudolf, along with both his wife Stéphanie and his mistress Mary Vetsera.1
A large number of studies have dealt with the inherited form of the disease. Apart from attention deriving from the discreditable means of transmission, interest grew because of the inexorable effect of syphilis-related abortions in reducing the population.2,3 Among the authors known for writing on this topic were Abraham Colles (1773-1843) and Charles-Paul Diday (1812-1894). Particularly worthy of mention is Jonathan Hutchinson, a physician at the London Hospital who, in 1861, described the so-called Hutchinson triad, consisting of nerve deafness, impaired vision, and notched incisors.2 Historical accounts of dermatology and venereology name Jean Alfred Fournier (1832-1914) as the physician who considered that congenital syphilis was the form that presented the most serious problems. That the mother transmitted the disease to her fetus during pregnancy was a late hypothesis and was not fully accepted until the latter part of the 19th century. It was Max Kassowitz who first stated in 1876 that infant mortality decreases with successive pregnancies in syphilitic mothers, a fact that was later demonstrated and accepted.3,4
In 1906, after serologic testing for syphilis was introduced, it was demonstrated that prior infection of the mother was required for mother-to-fetus transmission.4 Today, ever since the discovery of the culprit pathogen and the demonstration of its presence in fetal tissues (by Constantin Levaditi), the etiology of this disease is uncontested, as is evidence that fetal infection can be prevented by treating the mother.4
Under the assumption that a researcher chooses a method in keeping with premises he or she accepts as valid, an approach that provides a tool for analyzing real circumstances, we aimed to analyze the overall situation of congenital syphilis as it stands today. This critical review of the scientific literature on congenital syphilis seeks an effective way to enhance the modern reader's understanding of this disease. We will also discuss our extensive experience with patients in many different settings, from which we have gleaned a number of valuable lessons. Before drawing conclusions, in the first sections of this paper we will review the following aspects of congenital syphilis: the epidemiology of this form of the disease; the pathogen and its transmission; signs and symptoms of congenital syphilis; and its prevention, treatment and follow-up.
EpidemiologyAlthough preventive measures, such as the use of condoms, and effective treatments are available and relatively inexpensive, syphilis continues to pose health problems throughout the world, with 12 million new infections each year; nonetheless, the burden that congenital syphilis represents continues to be underestimated.5
In Latin American and Caribbean countries, where the prevalence rates are high in vulnerable groups, sexually active individuals are at risk. The 2003 AIDS Action project in Central America reported that the prevalence of syphilis in men who have sex with men ranged from 5% in Honduras to 13.3% in Guatemala, while the rate among professional sex workers was 6.8% in Honduras and 15.3% in El Salvador.6 In pregnant women, rates ranged from 0.4% in Panama to 6.2% in El Salvador.6 Incidence rates of congenital syphilis have been reported to be less than 0.1/1000 live births in Cuba but 4/1000 live births in Brazil.6,7 In Europe incidence rates of 1.9/100 000 live births in the United Kingdom,8 3.7/100 000 live births in Poland,9 and 12.1/100 000 live births in the Czech Republic10 have been reported. In Italy the incidence of 20/100 000 live births has been linked to infection in immigrant mothers (76%), with Eastern Europeans accounting for 44%, Africans for 20%, and South Americans for 12%.11
Experts who met in the Dominican Republic in May 2004 established a framework for eliminating congenital syphilis from Latin America and the Caribbean. The document in which they set out their conclusions included guidelines for developing surveillance and monitoring systems.12,13
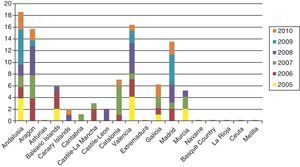
Cases of primary and secondary syphilis have been growing more numerous in the United States and many European countries, however. Until very recently, few infants were born with syphilis in high-income countries, but the last decade has seen a slight resurgence of the problem. The number of children diagnosed with syphilis in the UK rose from 2 in 1996 to 14 in 2005, and anecdotal reports suggest that the health care system is seeing many more cases of congenital syphilis than are counted; a pandemic may result if preventive strategies are not implemented.14 In Spain, the National Center for Epidemiology registered nearly 100 cases in the last 5 years (in sharp contrast with only 34 cases between 1998 and 2002) (Fig. 1).15 Sendagorta and coworkers16 have reported similar rates for Spain in their studies. Available statistics put the current incidence of congenital syphilis under 1/100 000 live births in this country, but little information is recorded on the national origin of mothers, other than incomplete data collected by the National Center for Epidemiology of the Carlos III Health Institute. Those figures are for 2009 and efforts are being made to obtain a complete report for 2010. The records presently tell us only that 3 mothers were from Romania and 1 was from Africa. The most recent figures also show that higher rates of congenital syphilis are found in communities where immigrants are more numerous.
Since the number of infants born with syphilis peaked at 4410 in 1991 in the United States, figures have fallen steadily; in 2004, 353 newborns had the disease (8.8/100 000 live births). Although congenital syphilis was very rare in Eastern Europe and the former Soviet Union in 1990, the numbers of infants born with the infection in the Russian Federation have been rising ever since: from 29 infected newborns registered in 1991, the number rose to 743 in 1999, reflecting in an increase in incidence from 0.9 to 8.5/100 000 live births.9,17–22
Syphilis is common among women of childbearing age in sub-Saharan Africa, with prevalence rates of 3.1% in Uganda,23 4.2% in Madagascar,24 6.6% in Ghana,25 and 8.3% in Zambia.25 Among pregnant women, the prevalence can be as high as 17%, and reinfection during pregnancy occurs in approximately 10%.23–25
The World Health Organization (WHO) estimates that the likelihood of vertical transmission is between 45% and 75%.5 The number of congenital syphilis cases varies annually from 700 000 to 1.5 million,26 and an estimated 420 000 to 600 000 perinatal deaths occur. About 40% of deaths correspond to stillbirths, and about 20% of live neonates fail to survive; the remaining 20% live,27 diagnosed with congenital syphilis.28
At the 2007 Women Deliver conference in London, the WHO launched a new global project to eliminate congenital syphilis as a public health problem. Sixty countries and international organizations dedicated to health, education and related services subscribed to the WHO's initiative. In keeping with that effort, we hope to raise awareness of the need for epidemiologic surveillance and monitoring of congenital syphilis among our younger colleagues, bringing to their attention the diagnostic and therapeutic challenges as well as the opportunities for widespread prevention.
The Pathogen and Its TransmissionTreponemes are macroaerophilic gram-negative bacteria measuring 6 to 20μm in length and 0.1 to 0.5μm in diameter. The T pallidum subspecies responsible for syphilis, which was sequenced in 1998, has an external membrane largely composed of lipids, with few proteins, a property which presents a hurdle to overcome in the development of diagnostic tests and effective vaccines.29
Mother-to-child infection usually occurs in utero; it is possible, though exceptional, for syphilis to be transmitted during childbirth. One infection scenario has the pathogen entering fetal circulation by first crossing fetal membranes and later infecting the amniotic fluid.30,31 Detection of T pallidum in amniotic fluid plays an important role not only in the antenatal diagnosis of syphilis but also in the assessment of its severity.32 Changes in the uterine cervix that are typical of pregnancy, such as hyperemia and friability, facilitate the pathogen's passage into the uterus, where it propagates.33
Statistics for mother-to-fetus transmission vary. It is estimated that 18% to 50% of infected mothers will pass the disease to their child,15 but the risk of infection and its severity decrease with successive pregnancies.34 The bacterial load in the mother decreases over time and risk of transmission is higher soon after infection than it will be later.35 However, we lack sufficient knowledge of the immunologic mechanisms that operate on the maternal-fetal unit to allow us to fully explain the natural history of congenital syphilis, its clinical progression, latency period, and relapses, or to accurately evaluate the results of treatment.15,34,36,37
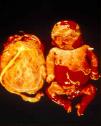
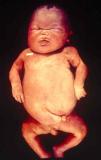
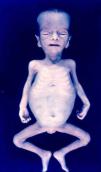
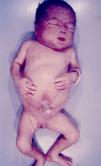
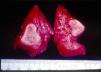
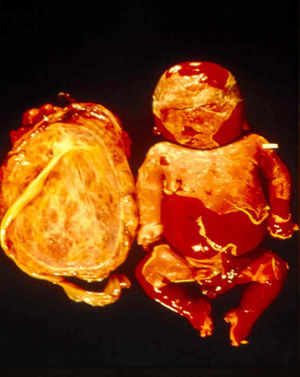
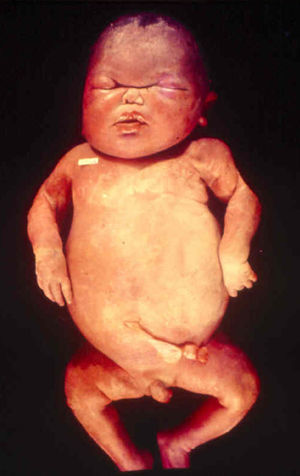
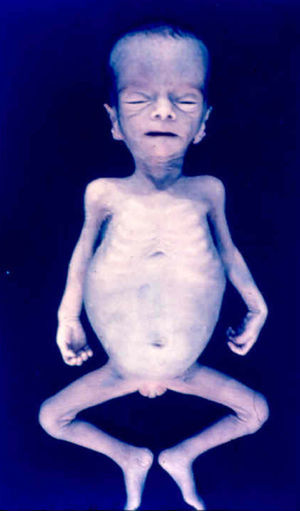
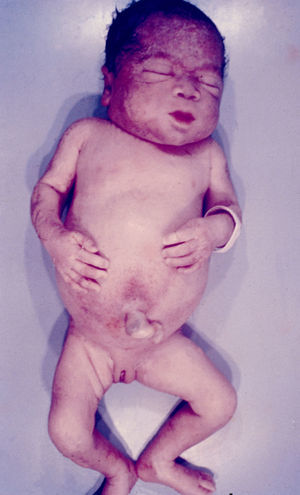
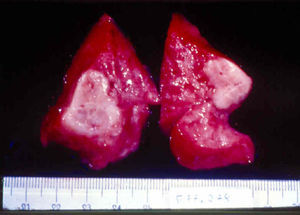
Signs and SymptomsAccording to available data and depending on severity of infection, the outcome of syphilis in the fetus is late spontaneous abortion (20%-40% of cases), stillbirth (20%-25%), prematurity with congenital syphilis (15%-55%), or full-term birth with congenital syphilis (40%-70%)15; thus, congenital syphilis can manifest, according to severity, as neonatal death, neonatal disease, or latent infection leading to later sequelae. Antenatal death is the most common outcome, occurring in 40% to 70% of pregnancies in mothers with untreated or inappropriately treated syphilis; most live newborns are asymptomatic but may develop manifestations later.15 Clinical signs can come early or late and their presentation varies greatly. Any fetal organ may be involved, but the disease most often affects the liver, kidneys, bone marrow, pancreas, spleen, lungs, heart, and brain. In the most characteristic histologic pattern of congenital syphilis, which resembles that of acquired syphilis, the disease manifests as obliterating endarteritis with a perivascular infiltrate of lymphocytes and plasma cells accompanied by intimal hyperplasia. Fibrosis and gummas are often present. Placental changes include focal villitis (villous proliferation) with necrosis as well as focal infiltration by maternal lymphocytes and plasma cells (Fig. 2). Villi are immature, enlarged and hypercellular. The umbilical cord may also show inflammatory changes and necrosis.38 Some studies have suggested an association between these features and prematurity, low birth weight, and small size in relation to gestational age; another association is spontaneous abortion, which may occur in the second trimester or at the beginning of the third.39–42
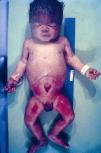
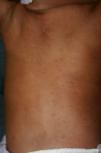
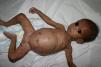
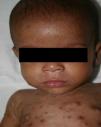
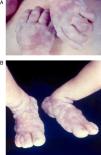
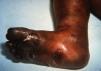
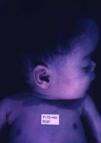
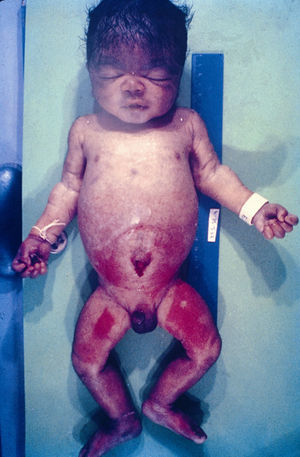
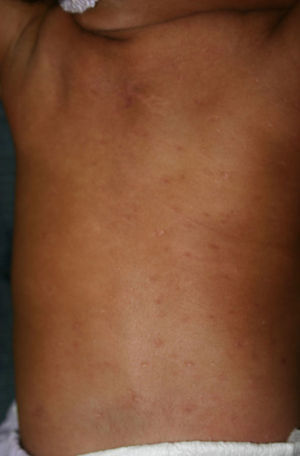
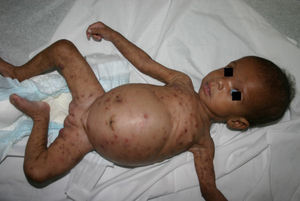
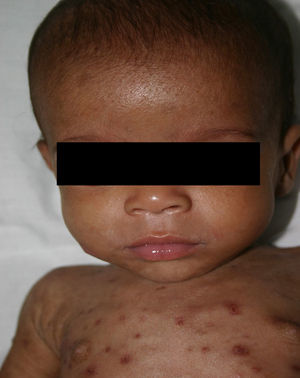
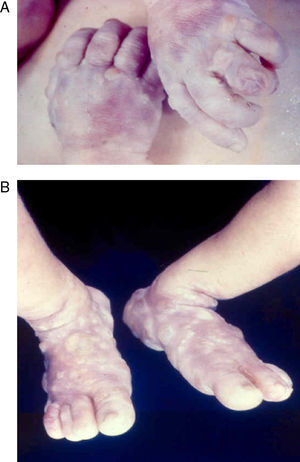
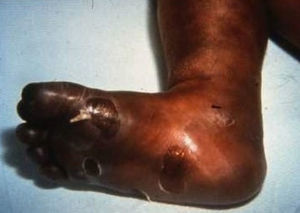
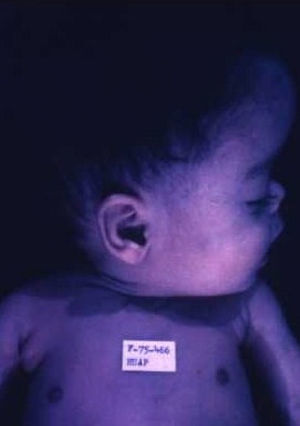
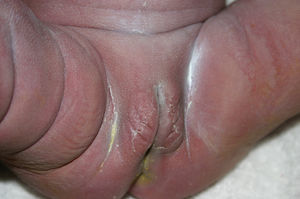
Early congenital syphilis can manifest up to the age of 2 years, but it is more common for symptoms to emerge during the perinatal period.43 As a large number of infected infants have no manifestations at birth, however, diagnosis is often delayed for several weeks. Disease may present with nonimmunologic hydrops fetalis, failure to thrive, kidney failure, hypothyroidism, myxedema, rhinitis, or pneumonia (Figs. 3-5).44 Affected infants typically have rhinitis with mucus that is rich in treponemes but may appear clear, purulent, or bloody; chondritis is also present, with consequent destruction of nasal cartilage and laryngeal involvement that may produce a cry that sounds hoarse.45 Maculopapular skin lesions (Figs. 6-9) develop in 30% to 60% of patients and are generally similar to those of adult secondary syphilis. A vesicular-papular rash may erupt, mainly on the palms and soles, varying in its extension. Lesions may be serous, purulent, or hemorrhagic. Ulcers and scabs develop later. These rashes are highly contagious46,47 (Figs. 10 and 11).
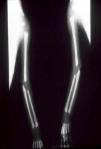
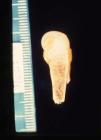
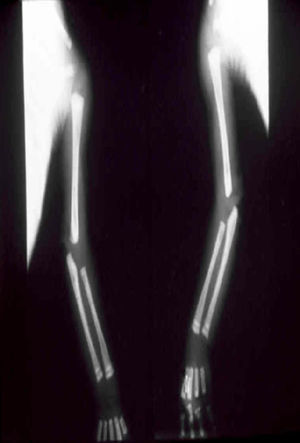
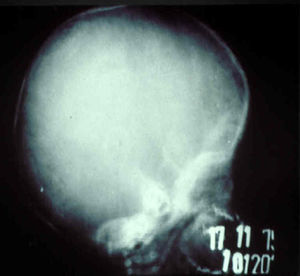
The early signs of nervous system involvement are meningitis and hydrocephalus, which may lead to mild to severe mental retardation or psychomotor disorders. The best known pulmonary complication is proliferative diffuse interstitial pneumonia, known as pneumonia alba.48 The kidneys are affected, and even in the absence of neurologic symptoms there may be cerebrospinal fluid (CSF) abnormalities. Most infants with apparently asymptomatic congenital syphilis have radiographic signs of osteochondritis or perichondritis, periostitis, and diaphyseal osteomyelitis (Figs. 12 and 13). The destruction of bone tissue may cause pain and fractures, leading to Parrot pseudoparalysis of an affected limb.48,49
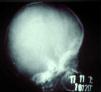
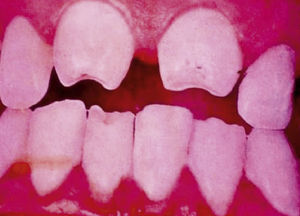
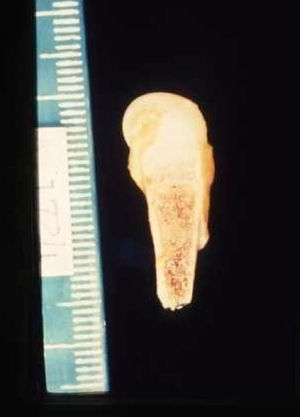
Late manifestations of congenital syphilis are similar to those seen in the adult,15,16 except for the rarity of cardiovascular involvement in the child. Malformations (clinical stigmas) may develop, either because infection occurred at a critical growth stage or as a direct result of disease activity.50 Examples of stigmas related to infection during a growth phase include saddle nose (the apparent enlargement of the distal part of the nose compared to a depressed bridge) (Fig. 14), saber shin, poorly developed or poorly enameled incisors, and malformed molars. The most widely known inflammatory sign is interstitial keratitis, which usually manifests in the child as blurred vision, photophobia, and excessive tearing. When these signs have resolved, syphilitic retinitis may become apparent. Eighth nerve deafness usually manifests late and may be either unilateral or, more often, bilateral. Nystagmus and balance disorders may accompany deafness. When interstitial keratitis occurs with Hutchinson's teeth (Fig. 15) and deafness, the patient has Hutchinson's triad, which is highly predictive of a diagnosis of congenital syphilis, conferring the characteristic features of the disease. Linear rhagades may form around natural orifices (Fig. 16).15,16 At some point, gummas may develop on the iris, ciliary body, or intrinsic and extrinsic muscles of the eye. Other organs, such as the lungs may also become compromised (Fig. 17). Bone and muscle involvement may take the form of osteoperiostitis or painless symmetrical inflammation of the knees, a condition known as Clutton's joints. Soft palate gummas are late manifestations, developing during childhood in 1% to 5% of patients with congenital syphilis; such gummas tend to progress more rapidly in children than they do in adults. A characteristic form of diaphyseal osteomyelitis compromises phalanges, metacarpals, and metatarsals. Depending on whether gummas are treated early and successfully, they may or may not lead to perforations, cysts, pathologic fractures and multiple deformities that are highly painful (Fig. 18). Late manifestations appear after the preschool years, between the ages of 5 and 14 years. Periostitis and diaphyseal osteomyelitis, mainly involving the tibias, are usually found together. As these long bones soften, they arch, in a process which will persist in the phase of bone condensation and thickening and lead to so-called saber shins.51,52 The most characteristic signs and symptoms of congenital syphilis are summarized in Table 1, with emphasis on mucocutaneous features.53
Signs and Symptoms of Congenital Syphilis.
| Antenatal phase |
| • Slow fetal growth |
| • Hepatosplenomegaly |
| • Echogenic bowel |
| • Hydrops fetalis |
| • Bowel obstruction |
| • Fetal death |
| Early phase |
| In neonates (resembles neonatal sepsis) |
| • Prematurity, low birth weight, very small for gestational age |
| • Vesicular rash, jaundicea |
| • Hepatomegaly, splenomegaly, anemia, fever, and septicemia |
| In infants and toddlers <2 y old (resembles secondary syphilis) |
| • Slow growth and developmental delays |
| • Ear-nose-throat manifestations: Rhinitis; perforation of nasal cartilage and the extension of cartilage growth toward the throat leads to laryngitis, snoring, or hoarse crying |
| • Mucocutaneous lesionsa: maculopapular rash; vesicular-papular rash, usually on the palms or soles (syphilitic pemphigus); condylomata lata (warts); fissures around the mouth, nose, or anus; petechiae; paronychia; eyebrow alopecia; brittle hair |
| • Ocular: uveitis, chorioretinitis, glaucoma |
| • Hematologic: anemia, high or low white blood cell counts, thrombocytopenia, disseminated intravascular coagulation |
| • Renal: nephrotic or nephritic syndrome with edema and ascites |
| • Central nervous system: leptomeningitis with vomiting, nuchal rigidity, separation of cranial sutures and bulging of the fontanelle. Progressive hydrocephalus |
| • Skeletal: osteochondritis, epiphysitis, periostitis, syphilitic osteomyelitis of the proximal phalanges (dactylitis), Parrot pseudoparalysis |
| • Other: hepatosplenomegaly, pancreatitis, multiple swollen lymph nodes, pneumonia alba, myocarditis |
| Late phase |
| In children older than 2 y (resembles tertiary syphilis) |
| • Mucocutaneous lesionsa: more pronounced fissures around the mouth, nose, or anus; gummas; perforation of the palate |
| • Ocular: interstitial keratitis, uveitis, glaucoma |
| • Auditory: eighth nerve deafness, associated with nystagmus and balance disorders |
| • Dental: Moon molars (mulberry-shaped), Hutchinson's teeth (peg-shaped upper central incisors with thinner notched lower edges) |
| • Neurologic: neurosyphilis that is asymptomatic in a quarter to a third of patients or symptomatic with tabes dorsalis (Charcot joint disease and perforating plantar ulcers), encephalitis (juvenile general paralysis), ataxia, sensory abnormalities, mood changes, convulsions, pupil abnormalities |
| • Skeletal: prominent forehead, short maxilla, arched palate, protruding mandible, saddle nose, saber shins, Clutton's joints (painful swelling), scaphoid (concave) scapula, Higoumenakia's sign (thickening of the sternal end of the clavicle) |
| • Other: cold hemoglobinuria |
Prenatal diagnosis of congenital syphilis is based mainly on ultrasound findings of characteristic signs, such as hepatosplenomegaly, hydrops fetalis with scalp edema, placental enlargement, and polyhydramnios.54,55 Fetal disease is diagnosed in 40% of pregnancies in mothers with early latent syphilis and in 10% with late latent syphilis.54 The combination of hepatosplenomegaly, enlarged placenta, bowel obstruction, and dilation of the fetal small bowel has also been observed.56
Treatment failure has also been reported in relation to these signs.57,58 Ultrasound assessment should be repeated because signs of disease normally disappear within 3 weeks of treatment. Fetal blood or amniotic fluid testing for trisomy might also be worthwhile,21 and needle aspiration of fetal ascites might demonstrate the presence of T pallidum. Aspiration of a fetal blood sample can help provide indirect prenatal evidence of disease, although these highly invasive procedures are usually inappropriate given that prenatal ultrasound findings of abnormalities plus a positive maternal serology will provide sufficient basis for a diagnosis of congenital syphilis.54
The US Center for Disease Control and Prevention (CDC) recommends systematic serologic screening, given that patients are usually asymptomatic.17,59 In Spain, screening for syphilis, toxoplasmosis, rubella, hepatitis and human immunodeficiency virus (HIV) infection usually takes place in the first trimester. Only the toxoplasmosis screen is repeated in the second trimester, if the first-trimester test was negative (if the mother is not immune). In the third trimester, hepatitis, HIV, and toxoplasmosis screening is repeated in mothers who had negative first-trimester results. All seropositive women should be considered infected unless there is a medical history of appropriate treatment and titers have decreased. Treatment should follow established protocols.17
The titers in syphilis serology may be unusually high, low, or fluctuating in HIV-positive patients.15,28
Serology or umbilical cord testing should not be ordered systematically after delivery: maternal serology is preferred, as levels will be undetectable in the umbilical cord if the mother's titers are very low or she was infected toward the end of pregnancy. The fluorescent treponemal antibody absorption IgM test was used for some time, but experience showed it to be of variable reliability, possibly attributable to high levels of maternal IgG or even because IgM immunofluorescence may be blocked. An elevated titer indicates infection. Immunofluorescence techniques seem to detect T pallidum quite accurately in samples from recent autopsy specimens.59–61
Newer diagnostic techniques are currently available. Some involve enzyme-linked immunosorbent assays, which can achieve a sensitivity of 100% and a specificity of 99% in pregnant women. Immunoblotting techniques have been used to detect IgM antibodies against T pallidum in fetal blood samples extracted by umbilical cord centesis.62–64 Polymerase chain reaction is also used to detect T pallidum in various fetal or neonatal fluids. These tools achieve high sensitivity, although results must be managed cautiously.65,66 Dark-field microscopic evaluation of neonatal nasal secretions or skin lesions can provide an immediate diagnosis.62 Histopathologic processing of these samples can also be useful. Silver-staining allows T pallidum to be visualized and immunohistochemistry will detect this pathogen. If the presence of the bacterium in clinical samples cannot be proven, the diagnosis must be based on clinical criteria and serology (Tables 1 and 2).
Guide to Interpreting Maternal and Infant Serology for Syphilis.a
| Nontreponemal Testing (VDRL, RPR, EIA) | Treponemal Testing (MHA-TP, FTA-ABS) | Interpretation | ||
| Mother | Child | Mother | Child | |
| – | – | – | – | No syphilis, or incubation period |
| + | + | – | – | Uninfected mother (false positive nontreponemal test results, with passive transfer of antibodies to child) |
| + | ± | + | + | Mother has syphilis, possibly transmitted to child; mother treated during pregnancy or mother with latent syphilis, with possible transmission to child |
| + | + | + | + | Recent or prepregnancy infection of the mother and possible transmission to child |
| – | – | + | + | Mother treated for syphilis before or early in pregnancy, or mother with Lyme disease or similar (false positive results) |
Abbreviations: EIA, enzyme-linked immunoassay; FTA-ABS, fluorescent treponemal antibody absorption test; MHA-TP, microhemagglutination assay for antibodies to Treponema pallidum; RPR, rapid plasma reagin test; VDRL, venereal disease research laboratory test.
We must underline the importance of new diagnostic tests that are easier to use in developing countries. Reagents, such as that used in the rapid plasma reagin test, for example, are stable at ambient temperatures. Self-contained tests for treponemes are available and require no electricity or additional equipment in the field. These tests are similar to the T pallidum particle agglutination assay with regard to sensitivity, specificity, and cost.
Other rapid diagnostic tests for syphilis include an immunochromatographic strip test and lateral flow chromatography using venous blood from a finger prick. These tests can be read in 10 to 15minutes even outside a laboratory setting; good results for such techniques were reported from a field study in Mozambique.67,68
Prevention, Treatment, and Follow-upThe strategies available for preventing congenital syphilis fall into 3 categories: a) case finding and treatment of all cases of primary and secondary syphilis in the community and follow-up of contacts, as well as rescreening of high-risk pregnant women after initial serology (to reduce the incidence of early syphilis in women of childbearing age; b) follow-up of seropositive cases by investigative methods and screening in selective high-risk groups (to reduce the prevalence of latent syphilis); and c) routine prenatal testing (which is the best defense against congenital syphilis). Testing should be repeated to detect infections that might occur during pregnancy.63,64
Summarizing CDC recommendations,17 pregnant women who are at high risk of syphilis infection, who reside where the prevalence is high, who have not previously been tested, or who were seropositive on first-trimester testing should be retested at the beginning of the third trimester (at 28 weeks of gestation) and on delivery. As mentioned, strict preventive measures have been put into effect in countries like the Russian Federation. Some countries screen all women after delivery. Neonates should not be taken from the hospital until the mother has been tested for syphilis at least once during pregnancy and, preferably, tested after delivering. Any woman who gives birth to a stillborn fetus should also be tested, and all pregnant women with syphilis should also be tested for HIV infection (Table 3). Treatment for syphilis during pregnancy consists of administering penicillin at doses appropriate for the gestational stage. Some authors recommend a second intramuscular injection of benzathine penicillin G a week after the first dose in early acquired syphilis (infection within the past year). We must also mention the possibility of a Jarisch-Herxheimer reaction, which occurs in up to 45% of pregnant women who are treated. The aqueous crystalline penicillin G solution for parenteral infusion is preferred in the interest of ensuring adequate CSF concentrations. If therapy is interrupted for more than 24hours, it should be restarted. Tests and treatments recommended for pediatric patients with congenital syphilis are summarized in Table 3, although we must stress the important situations affecting single-dose treatment of a neonate. The first scenario would involve neonates with normal findings on physical examination and serum titers 4-fold lower than the mother's titer or at least not exceeding it. The second would involve neonates whose mothers were appropriately treated within the 4 weeks before delivering and have shown no signs of reinfection or relapse. In these infants, further testing is unnecessary and a single intramuscular injection of 50 000 IU/kg benzathine penicillin G is the recommended treatment. A final consideration involves the cases of neonates with normal findings on physical examination and serum titers equal to the mother's or 4-fold lower, and whose mothers were appropriately treated and have had stable titers on follow-up: some specialists nonetheless recommend that the infant receive single-dose treatment with 50 000 IU/kg benzathine penicillin G, particularly if the clinician cannot count on being able to follow the case.
Evaluation and Treatment of Infants With Congenital Syphilis.
| Recommended Tests, Depending on Clinical Findings, and Treatments | 1. Infants With Proven or Highly Probable Congenital Syphilis | 2. Infants With a Normal Physical Examination and Serum Titersa and a Mother Who Was Not Treated, Not Treated Appropriately, or Treated <4 Weeks Before Delivery | 3. Children Aged >1 mo With Reactive Serology—Should Have Maternal Serology and History Reviewed to Distinguish Congenital From Acquired Syphilisb |
| Clinical manifestations | X | X | |
| Eye-ear tests | X | X | |
| Pathology and bacteriology (placenta, umbilical cord, body fluids)c | X | ||
| RPR and VDRL serology | X | X | |
| IgM assays in serum: FTA-ABS, EIA, immunoblottingd | X | ||
| CSF (VDRL, cell count, and protein values) | X | X | |
| Complete blood count | X | X | X |
| Liver-function tests | X | X | |
| Long-bone radiographs | X | X | X |
| Chest radiograph | X | X | |
| Cranial ultrasound | X | X | |
| Abdominal ultrasound | X | ||
| Treatment | Aqueous penicillin G 100 000–150 000 IU/kg/d (50 000 IU/kg IV every 12h during the first 7 d of life and every 8 h thereafter for a total of 10 d) or procaine penicillin G (50 000 IU/kg in a single IM injection for 10 d) | As in the previous scenario, or benzathine penicillin G 50 000 IU/kg in a single daily IM injection | Aqueous penicillin G 200 000–300 000 IU/kg/d (50 000IIU/kg IV every 4-6 h for 10 d) |
Abbreviations: CSF, cerebrospinal fluid; EIA, enzyme-linked immunoassay; FTA-ABS, fluorescent treponemal antibody absorption test; IM, intramuscular; IV, intravenous; RPR, rapid plasma reagin test; VDRL, venereal disease research laboratory test.
Still summarizing guidelines and recommendations in reviews,17,66 all seropositive children or infants whose mothers were seropositive at delivery should be followed with exhaustive physical examinations and serologic testing (e.g., nontreponemal tests every 2-3 months until titers are nonreactive or have decreased 4-fold). Nontreponemal antibody titers should fall when the infant is 3 months old and should be nonreactive at 6 months if the infant was not infected (i.e., if a positive titer was the result of transfer of maternal IgG antibodies) or was infected and appropriately treated. If titers remain stable or increase between 6 and 12 months of age, the CSF should be tested and the infant should be treated for 10 days with parenteral benzathine penicillin G at recommended doses.
Summarizing a recent review of testing,62 if a treponemal test is nonreactive at 18 months, further evaluation or treatment will be unnecessary. If titers are reactive at this time, however, the child should be reevaluated fully and treated for congenital syphilis. Abnormal CSF findings on initial testing indicates a need for lumbar punctures approximately every 6 months until results are normal.
Guidelines17,60 also state that special care should be taken with all children who require treatment for syphilis but who have a history of allergy to penicillin or who experience an apparent allergic reaction to the drug. Such children should first be desensitized and then treated with penicillin at the recommended doses. Although we still lack evidence to support the efficacy of other antibiotics in the treatment of syphilis, age- and weight-adjusted dosing regimens have been recommended for some. Ceftriaxone is an example. For infants aged at least 30 days, a single daily intravenous or intramuscular injection of 75mg/kg of this drug has been suggested. Treatment should be continued for 10 to 14 days; however, this dose may have to be adjusted in accordance with an infant's actual weight. For older children, a single daily dose of 100mg/kg is recommended. Premature infants who have no other clinical evidence of infection can be given intravenous ceftriaxone at doses adjusted for age and birth weight. A strict CSF testing regimen should be undertaken in all cases.
We lack information on whether children with congenital syphilis and HIV-coinfected mothers need to be evaluated, treated, and followed for syphilis in ways that are different from the protocols recommended for other children.17,62,66
Finally, we would like to mention that it would be useful to have an effective vaccine, which would serve to generally fight the disease by preventing long-term complications, reinfection, and further transmission; it might also contribute to reducing HIV transmissibility.69
DiscussionThe incidence of congenital syphilis appears low in Spain and other European countries according to available epidemiologic statistics, but it is very likely that the real incidence is underestimated. The high incidence of this disease in Italy is mainly attributable to infected immigrant mothers from Eastern Europe and Africa.11 Information for Spain is scarce, but a similar pattern can be expected.
We have concluded that the birth weights and gestational ages of infants born to seropositive mothers in the Dominican Republic and Brazil tend to be lower than those reported for the offspring of other seropositive mothers described in the literature. Epidemiologic data supporting this conclusion comes from the Dominican Republic's office of the Secretary of State for Public Health and Social Services (SESPAS), within the ministry of the same name, and Brazil's Information System for Notifiable Diseases (SINAN), an organism of that country's Ministry of Health. We also note that there is supporting clinical data from the Hospital Materno-Infantil de Santo Domingo, in the Dominican Republic, and the pathology department of the medical faculty of Universidad Federal Fluminense in Niterói, Brazil. The available data suggest to us that only 32% of infected neonates show overt signs of congenital syphilis. For 16% the only sign is seropositivity for IgM antibody; 12% have positive venereal disease research laboratory test findings in CSF, and 8% have serum titers that exceed their mothers’.
The importance of prenatal detection of syphilis is well-documented9 and the test is highly cost-effective even in countries with a low prevalence of the disease.70 Most UK and US centers recommend syphilis testing for all women at the start of pregnancy, at 28 weeks, and at delivery if the woman is at high-risk of infection.14 The Russian Federation tests at the start of pregnancy and at 21 and 36 weeks.19
Other countries, such as France and Italy have passed laws that require free testing (Decrees 92-143/2/1992 and 10/09/1998, respectively), although coverage only provides for first-trimester screening.70,71 Systematic screening for syphilis is also only done in the first semester in Spain, where the syphilis test is included along with serology for toxoplasmosis, rubella, hepatitis, and HIV. The same protocol is used in Italy, where higher prevalence rates are currently observed in immigrant populations from Eastern Europe.
Vigilance must continue to determine whether the prevalence of seropositivity for syphilis continues to rise among immigrant mothers in Spain, as in Italy, although the risk of delivering an infected infant should be the same in immigrants and other mothers given that all have equal access to national health care. However, we believe that higher risk may derive from the following factors: previous contact with multiple sexual partners, prostitution, drug addiction, HIV infection, and immigration, especially if the mother comes from a country where syphilis is endemic. When such risk can be identified, serologic testing should be repeated at 28 weeks and at delivery.
For developing countries, better programs to bring congenital syphilis under control—through more effective prenatal screening and treatment of infected infants—are imperative. Necessary actions would include campaigns to encourage early prenatal care, the provision of information for both women and men on the benefits of being screened for acquired syphilis and treating it early, and the modification of high-risk sexual behaviors. Condom use should be promoted. Decentralized implementation of such schemes would be highly useful. The rapid tests described above should be used, and treatment should start on the same day T pallidum is detected. Sexual partners should also be treated whenever possible. At the end of pregnancy, serology should be repeated in the interest of screening for reinfection and, naturally, all new mothers who did not receive prenatal care should be tested. Infants with proven, probable, or suspected disease should also start treatment for congenital syphilis.
When mass antibiotic treatment of all people aged 15 to 49 years was tried in an area of Uganda, 1-g doses of azithromycin significantly reduced the prevalence of syphilis in the population.72 Trials in Uganda and Tanzania have demonstrated that azithromycin alone or in combination with benzathine benzylpenicillin G was at least as effective as benzathine benzylpenicillin G alone for treating early syphilis. This strategy offers considerable advantages, in terms of adherence, in comparison with traditional therapy based on injectable penicillin. These findings should be examined carefully, however, and the possible use of this drug in pregnant women considered cautiously, given recent reports of resistance to azithromycin.73
The 1998 sequencing of the genome of T pallidum raised the hope of a vaccine, and a recent review of the literature concluded that one should be feasible within the next decade; funding is lacking, however.74
Simpler ways to diagnose syphilis, and in particular congenital syphilis, are still needed. Noninvasive diagnostic techniques—based on saliva samples, for example—would be useful, as would an easy way to predict which neonates will require later treatment and follow-up because of antenatal exposure resulting from inappropriate treatment of the mother.
Participants at the May 2006 World Health Assembly adopted a global strategy for eliminating congenital syphilis, emphasizing the interest of including this disease in the assembly's broader initiatives to prevent and control other sexually transmitted infections.
Thus, efforts to screen for syphilis should aim for synergies with HIV-AIDS programs for preventing mother-to-child transmission of the virus.75
The resources for eliminating congenital syphilis are currently inadequate and there is urgent need to create a working group to raise global awareness of the tragedy of our present situation. That effort, which targets the health of mothers and children, could help to meet the Millennium Development Goals set out by the United Nations in their campaign to eradicate extreme poverty.
ConclusionsFrom the clinician's point of view, this article emphasizes the highly variable clinical picture of congenital syphilis and shows how difficult it can be to diagnose this disease, especially if one is unfamiliar with its management.
We emphasize the following conclusions:
- •
All pregnant women should be screened early in pregnancy. At 28 weeks and at delivery we should retest women at high risk of infection or those coming from countries where the incidence of syphilis is high.
- •
Women who have not received prenatal care should be examined before they are discharged from the maternity ward.
- •
The mothers of all infants stillborn after 20 months of gestation should be tested for syphilis.
- •
Approximately two-thirds of infants with congenital syphilis have no symptoms at birth; therefore, pediatricians and dermatologists should watch for possible cases.
- •
Penicillin continues to be the most effective treatment for syphilis.
- •
All infants with proven or probable congenital syphilis should be treated as soon as possible and should be followed to confirm that treatment was effective.
- •
Developing countries should promote prenatal care as well as the benefits of screening for syphilis and treating it early. Campaigns to encourage the use of condoms and promote the modification of high-risk sexual behaviors should be undertaken in such countries.
We believe that reviews like this one are of particular importance in Spain, where few clinicians have experience with this disease, although considerable effort to compile epidemiologic and clinical data is now under way.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Salvador de Mateo Ontañón of the surveillance division of the Spanish National Center for Epidemiology (Carlos III Health Institute, Madrid) for his assistance with epidemiologic data.
We are also grateful to Rafael Alcántara Cáceres of the Instituto de Dermatología y Cirugía de la Piel (IDCP) in Santo Domingo, Dominican Republic; it is always a pleasure to work with Dr. Alcántara, who is also a member of the Latin American Union Against Sexually Transmitted Diseases, an organism of the Pan-American Health Organization and the World Health Organization.
Please cite this article as: Rodríguez-Cerdeira C, Silami-Lopes VG. Sífilis congenital en el siglo XXI. Actas Dermosifiliogr. 2012;103:679-693.