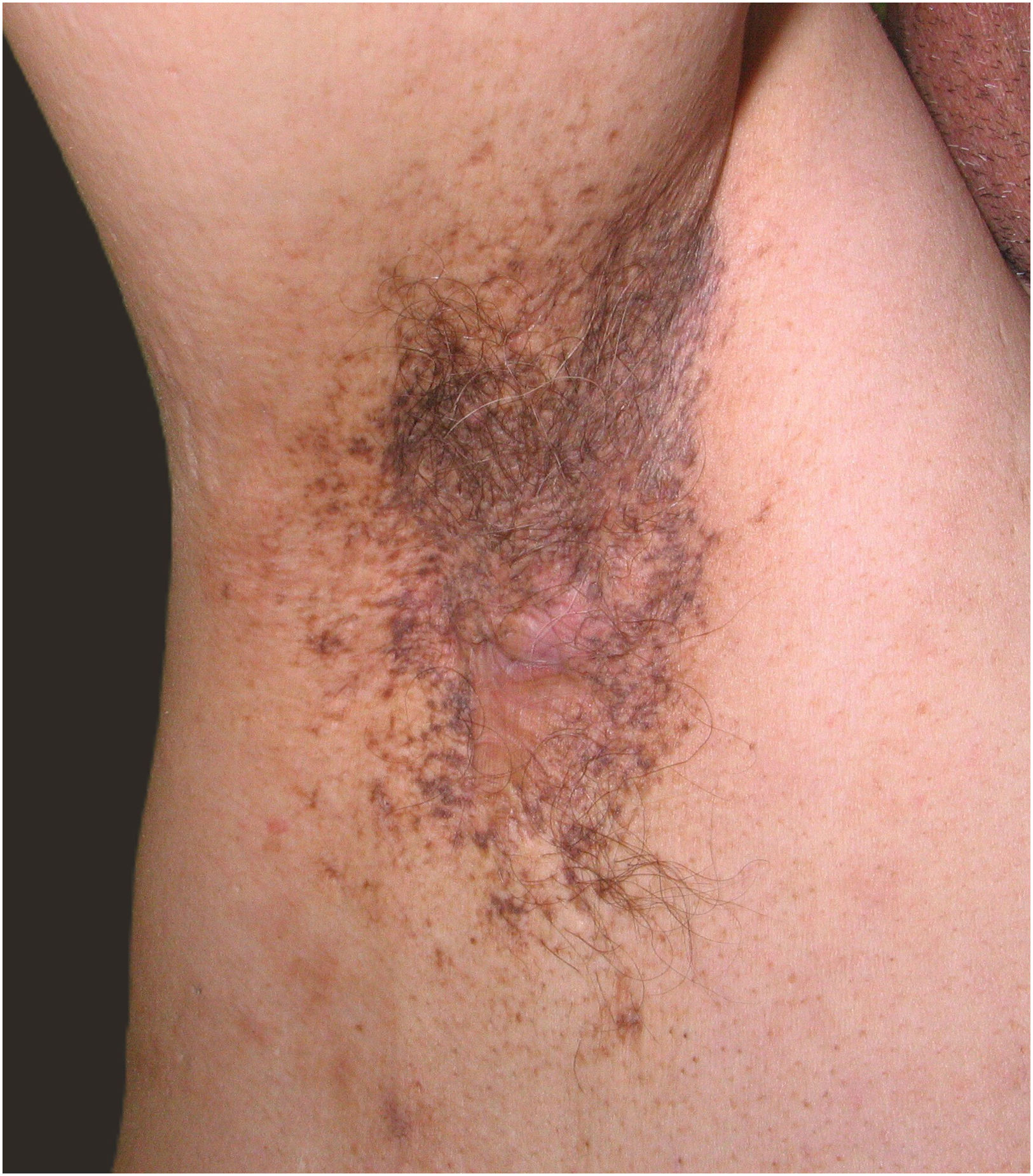
Dowling-Degos Disease (DDD) is a benign condition characterised by reticulate hyperpigmentation and follicular defects. Skin lesions are typically asymptomatic and manifest over the flexures during the 2nd to 5th decades of life, without sex predominance. Secondary features of DDD include pitted facial and perioral scars and comedo-like lesions. Although various associations with DDD have been described such as arthritis or squamous cell carcinoma, the most well-known is the co-occurrence with hidradenitis suppurativa (HS).1
The pathogenesis of DDD involves various genes, including KRT5 (keratin 5 gene), POGLUT1 (protein O-glucosyltransferase 1), POFUT1 (protein O-fucosyltransferase 1), and PSENEN (presenilin enhancer protein 2 gene), which are involved in melanosome transfer, melanocyte, or keratinocyte differentiation. The coexistence of familial cases of HS and DDD is possible since HS can share mutations in POGLUT1, POFUT1, and PSENEN, whose protein products comprise the γ-secretase complex and the Notch signalling pathway.2
Diagnosis of DDD is based on clinical and histopathological findings, with hematoxylin–eosin revealing filiform or antler-like epidermal downgrowth extending to the superficial dermis and hair follicle walls. Other microscopic changes may include dermal fibrosis, elongated rete ridges, horn cysts, basilar and dermal melanosis, and perivascular lymphocytic infiltrate.3
We retrospectively reviewed a multicentric cohort of 32 patients from Spain with concomitant HS and DDD, extending a case series of 15 patients previously published by Agut-Busquet et al.4 The primary endpoint of the study was to describe demographic and clinical characteristics. Secondly, we assessed possible differential factors between Group 1 (DDD with concomitant HS) (Fig. 1) versus Group 2 (isolated HS) (Fig. 2). A punch biopsy was performed in flexural areas to confirm DDD diagnosis in all suspected cases. Statistical analysis was performed using parametric tests such as chi-square and t-tests. Significance level was set at p<0.05. For multiple comparisons, p-values were corrected using the Bonferroni correction. Our results are summarised in Table 1.
Comparison between patients with associated DDD vs isolated HS.
| Concomitant HS and DDD(N=32) | HS group(N=638) | p-Value | |
|---|---|---|---|
| Sex (female) | 18 (56.3%) | 291 (45.6%) | 0.239 |
| Body mass index | 27.9±5.2kg/m2 | 29±6.74kg/m2 | 0.081 |
| Active smoker or former smoker | 27 (84.4%) | 409 (68.4%) | 0.056 |
| Fitzpatrick skin type (I, II, III, IV, V, VI) | 0, 0, 31 (96.9%), 1 (3.1%), 0, 0 | 0, 5 (0.8%), 575 (97.6%), 8 (1.4%), 1 (0.2%) | 0.325 |
| Family history of HS | 24 (75%) | 228 (40.4%) | <0.001 |
| Age of onset for HS | 16.8±6.1 years | 23.1±10.6 years | 0.002 |
| Canoui-Poitrine phenotype (LC1, LC2, LC3) | 10 (31.3%), 16 (50%), 6 (18.8%) | 132 (41.1%), 74 (23.1%), 115 (35.8%) | 0.136 |
| Hurley (I, II, III) | 5 (15.6%), 17 (53.1%), 10 (31.3%) | 243 (41.6%), 188 (32.2%), 153 (26.2%) | 0.443 |
| Type of HS lesions | |||
| Nodules | 27 (84.4%) | 302 (47.5%) | <0.001 |
| Abscesses | 6 (18.8%) | 151 (23.8%) | 0.507 |
| Fistulas | 14 (43.8%) | 250 (39.4%) | 0.626 |
| Comedones | 4 (12.5%) | 75 (11.9%) | 0.914 |
| Scars | 14 (43.8%) | 103 (16.3%) | <0.001 |
| Pyoderma gangrenosum | 2 (6.3%) | 12 (1.9%) | 0.092 |
| Epidermal cysts | 15 (46.9%) | 85 (13.3%) | <0.001 |
| Location of HS lesions | |||
| Nape | 9 (28.1%) | 41 (6.4%) | <0.001 |
| Axillae | 24 (75%) | 303 (47.5%) | 0.002 |
| Trunk | 22 (68.8%) | 73 (11.4%) | <0.001 |
| Groins | 23 (71.9%) | 270 (42.3%) | 0.001 |
| Genital | 14 (43.8%) | 82 (12.9%) | <0.001 |
| Glutei | 13 (40.6%) | 118 (18.5%) | 0.002 |
| Perianal | 5 (15.6%) | 71 (11.1%) | 0.434 |
| Pitted facial scars | 7 (23.3%) | Not reported | – |
| History of sacral cyst | 15 (46.9%) | 150 (25.7%) | 0.009 |
| Inflammatory bowel disease | 1 (3.1%) | 20 (3.1%) | 0.998 |
| Arthritis | 0 | 20 (3.1%) | 0.309 |
| Squamous cell carcinoma | 0 | Not reported | – |
| Mental health disease | 11 (34.4%) | 67 (10.5%) | <0.001 |
| Ongoing treatment with adalimumab | 3 (9.4%) | 41 (6.4%) | 0.511 |
The bold values correspond to statistically significant results.
We present the most extensive case series of DDD with concomitant HS. Former studies on isolated HS have shown a female-predominance with a 3:1 sex ratio and a prevalence of current or former smokers >70%.5,6 In our series, sex, smoking habits, and BMI did not show statistical differences between Groups #1 and #2. Most patients had a Fitzpatrick skin type III, the most prevalent skin type in our region. As expected for an autosomal inheritance, a family history of DDD was reported in 50% of patients in Group #1. Moreover, the age of onset for HS resulted significantly earlier in Group #1. The Canoui-Poitrine phenotype revealed an outsized proportion of LC2 (50%) in Group #1, whereas the predominant phenotype in Group #2 was LC1 (41.1%) followed by LC3 (35.8%). The Hurley stage demonstrated a higher proportion of stage II–III patients in Group #1 (84.4% vs. 58.4%), suggesting a more severe HS in patients affected with concomitant DDD, although not statistically significant after applying Bonferroni correction. Regarding the type of lesions, nodules, epidermal cysts and scars were more prevalent in Group #1 vs #2 (84.4% vs 47.5%, 43.8% vs 16.3%, 46.9% vs 13.3%), respectively. Additionally, Group #1 presented a higher involvement of all anatomical areas except for the perianal region. The study of comorbidities evidenced a 2-fold history of pilonidal sinus in Group #1 vs Group #2. The prevalence of mental disorders including anxiety, personality disorder, depression, bipolar disorder, and substance abuse was higher in Group #1 (34.4% vs 10.5%). Some of these may be attributed to a higher severity in Group #1 along with the aesthetic impact of DDD lesions.7
Limitations of this study include its retrospective nature and small sample of patients in Group #1 due to the rarity of the disease. Additionally, the control group included patients from a monographic HS clinic from a tertiary referral center, thus representing a more severe subset of patients.
In conclusion, we found a distinct profile between patients with concomitant HS and DDD vs isolated HS. The former exhibiting a stronger family history of HS, an earlier onset of the condition and a greater prevalence of pilonidal sinus and mental disorders. Furthermore, patients from this group showed a higher proportion of nodules, epidermal cysts, and scaring.
Conflicts of interestPGS declared to have received honoraria from Novartis, Celgene and UCB for participation on advisory boards, conferences, and as investigator in clinical trials.
VES declared to have received honoraria from Abbie, Lilly, LEO Pharma, Novartis and Sanofy Genzyme for participation on advisory boards, conferences, and as investigator in clinical trials.
JR declared to have received honoraria from Abbvie, Novartis, Almirall, Janssen, UCB, Leo Pharma and Celgene for participation on advisory boards, conferences, and as investigator in clinical trials.
AM declared to have acted as a consultant, advisory board member and investigator, and received honoraria from AbbVie, Amgen, Janssen Cilag, LEO Pharma, Lilly, Novartis, L’Oreal, Sandoz, Sanofi and UCB.
JCP and EAB declared conflicts of interest whatsoever.