Facial lentigo maligna melanoma can be a diagnostic challenge in daily clinical practice as it has similar clinical and morphological features to other lesions such as solar lentigines and pigmented actinic keratoses. Confocal microscopy is a noninvasive technique that provides real-time images of the epidermis and superficial dermis with cellular-level resolution. We describe 3 cases of suspected facial lentigo maligna that were assessed using dermoscopy and confocal microscopy before histopathology study. In the first case, diagnosed as lentigo maligna melanoma, presurgical mapping by confocal microscopy was performed to define the margins more accurately. In the second and third cases, with a clinical and dermoscopic suspicion of lentigo maligna melanoma, confocal microscopy was used to identify the optimal site for biopsy.
El diagnóstico del lentigo maligno melanoma facial constituye un reto en la práctica clínica habitual debido al solapamiento de ciertas características clínicas y morfológicas con lesiones como lentigos solares o queratosis actínicas pigmentadas. La microscopia confocal es una técnica no invasiva que permite obtener imágenes en tiempo real de la epidermis y la dermis superficial con resolución a nivel celular. En esta serie se describen 3 casos de lesiones faciales sospechosas de lentigo maligno, evaluadas por dermatoscopia y microscopia confocal antes de realizar el análisis histopatológico. En el primer caso, con diagnóstico de lentigo maligno melanoma, se realizó un mapeo prequirúrgico mediante microscopia confocal, para delimitar los márgenes con mayor precisión, y en el segundo y el tercer caso con sospecha clínica y dermatoscópica de lentigo maligno melanoma se identificó la zona óptima para realizar la biopsia.
Reflectance confocal microscopy is a noninvasive technique that uses a low-power laser system to produce in vivo cellular-resolution images of the skin to the depth of the papillary dermis.1,2 It has been shown to improve diagnostic accuracy in melanoma and nonmelanoma skin cancer, particularly in the case of difficult-to-diagnose lesions.3–7
Facial skin has particular histologic characteristics related to the prominence of adnexal structures, flattening of rete ridges, and a varying degree of elastosis in the dermis. Dermoscopic examination of pigmented lesions on the face rarely shows the pigment network seen in lesions in other parts of the body. What is generally observed is a wide-meshed pseudonetwork formed by numerous hair follicles and sweat gland openings.8 This pseudonetwork can be seen in all types of pigmented facial lesions, whether melanocytic or nonmelanocytic.8–10 Diagnosis of facial lentigo maligna (LM) and facial lentigo maligna melanoma (LMM) can often be a challenge as the clinical and dermoscopic features overlap with those of other lesions such as solar lentigines and pigmented actinic keratoses.11 Similarly, it is not uncommon for lesions with equivocal clinical and dermoscopic features, or even benign lesions, to be diagnosed as LM/LMM. Tools with improved diagnostic accuracy are therefore essential to enable the early detection of malignant lesions and avoid the unnecessary excision of benign lesions.12
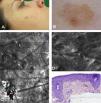
Case 1The first patient was a 37-year-old woman with a history of multiple melanoma and familial melanoma. She was a carrier of the high-risk CDKN2A mutation, 358delG. At a check-up visit, we observed a 7 x5-mm pigmented lesion on the right cheek that had been present for less than 6 months. Dermoscopy showed a polychromatic (light brown, dark brown, and gray) lesion with asymmetric pigmentation, a gray annular-granular pattern, and a pigmented pseudonetwork. The lesion therefore had dermoscopic features of both LM/LMM and pigmented actinic keratosis. Examination of the lesion with confocal microscopy showed stratum corneum disruption, disturbance of epidermal architecture (atypical honeycomb pattern), and atypical keratinocytes in the suprabasal layer, with several small dendritic cells (<20μm); there were no signs of perifollicular distribution or pagetoid invasion. The superficial dermis showed a moderately refractile stroma with thickened bundles, consistent with solar elastosis (Fig. 1). These findings were strongly suggestive of actinic keratosis and confocal microscopy was used to identify the optimal site for biopsy, which confirmed the diagnosis. The lesion was treated with adapalene 0.1% daily for a month and the follow-up results have been favorable.
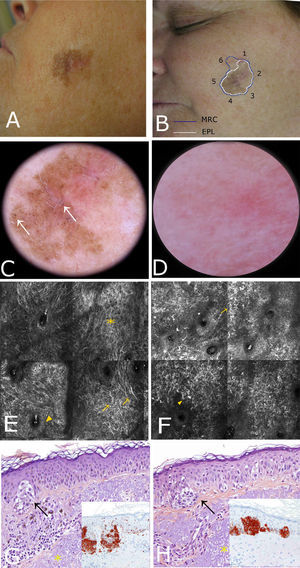
Case 1. A, Photograph. B, Dermoscopic image showing a pseudonetwork with gray dots and a more intensely pigmented eccentric area with focal perifollicular hyperpigmentation. C, Confocal submosaic (1300×800μm) showing an irregular refractile amorphous material (
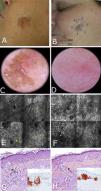
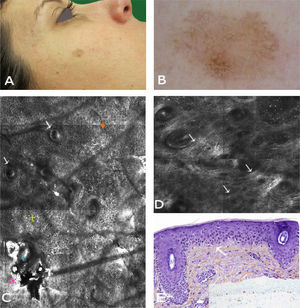
A 56-year-old woman with no relevant past history was referred to our center for presurgical evaluation of a lesion on the left cheek; a histology report from another center indicated a diagnosis of in situ melanoma, LM type. Physical examination revealed a brown plaque measuring 21×17mm, with a 6-mm central scar, poorly circumscribed borders, areas of brown pigment and obliteration of follicular openings, and sparsely pigmented areas at the periphery; there were no clear dermoscopic signs of LMM (Fig. 2A). Because accurate identification of margins is crucial for the presurgical evaluation of LMM, and the edges of the lesion were not clearly visible on dermoscopy, we used confocal microscopy to map the adjacent areas (Fig. 2E). The microscopic findings showed signs compatible with LMM in both the pigmented area and an achromic area adjacent to the lesion. Using this information, we mapped the presurgical margins and excised the tumor, which finally measured 28x20mm. Histologic examination showed the margins to be clear and the surgical defect was repaired with a rotation flap. Focal recurrence was observed 6 months later and cleared with simple excision. There were no signs of recurrence at the 2-year follow-up.
Case 2. A, Photograph showing a pigmented lesion (21×17mm) with irregular borders and a central scar on the left cheek. B, Clockwise mapping of the periphery of the lesion, showing the limits seen by dermoscopy (white line) and confocal microscopy (blue line). Microscopic examination revealed an achromic area with signs compatible with LM. C, Dermoscopic image showing perifollicular pigment and obliteration of the follicular openings (
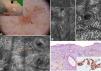
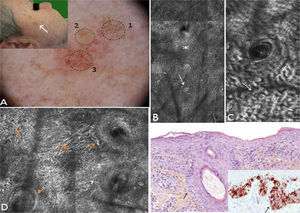
A 62-year-old man with no relevant history consulted for an erythematous lesion measuring 10x7mm in the right malar region. Dermoscopy revealed 3 distinct areas: an upper pigmented area with a gray annular-granular pattern; a central papule; and a sparsely pigmented lower area with red rhomboidal structures, a recently described dermoscopic feature of LM.10 The dermoscopic findings in our patient were not conclusive, and the differential diagnosis included LM/LMM, pigmented actinic keratosis, and solar lentigo. We therefore decided to examine the lesion by confocal microscopy. The findings are described in Fig. 3. The examination ruled out malignancy in area 1, showed signs of seborrheic keratosis in area 2, and signs of LM in area 3. A punch biopsy of area 3 confirmed the diagnosis of LM, which was corroborated by immunohistochemistry. The lesion was completely excised. Histologic examination confirmed the presence of foci of melanocytic hyperplasia, with no evidence of LM. This diagnosis was also corroborated by immunohistochemistry.
Case 3. A, Photograph and dermoscopic image showing pigmentation with a gray annular-granular pigmentation in area 1, a central papule in area 2, and incipient red rhomboidal structures at the bottom of area 3. B, Confocal submosaic (1000×500μm) of the epidermis in area 1 showing a typical honeycomb pattern with isolated highly refractile cells without signs of atypia. C, Confocal submosaic (350×600μm) of the epidermis in area 2 showing epithelial cords compatible with seborrheic keratosis. D, Confocal submosaic (800×900μm) of the epidermis in area 3 showing a destructured honeycomb pattern (
Confocal microscopy is an emerging technique for clinical research in skin cancer. Its main advantage is that it offers a unique opportunity to evaluate skin morphology in vivo. This “virtual biopsy” avoids unnecessary excisions, and while biopsies and excisions are not complex procedures, the associated morbidity and cost of histologic examination are important aspects from the perspective of both the patient and the health care system.
Dermoscopy has a sensitivity of 83.2% and a specificity of 85.5% for melanoma.13Confocal microscopy has been found to have higher sensitivity (97.3%) and similar specificity (83%).14 Nonetheless, because the technique requires special training and is more time-consuming than dermoscopy, it is not indicated for initial evaluation but rather for further analysis of lesions with equivocal findings on dermoscopy. One limitation of confocal microscopy is that it is not a reliable method for analyzing depth of invasion or lesions involving the reticular dermis as its maximum penetration depth is between 350μm and 500μm, which corresponds to the papillary dermis. Also, as with any imaging technique, interpretation of results depends on the observer's training, although automated diagnostic imaging systems are currently being developed.
Multiple studies have demonstrated that confocal microscopy improves diagnostic accuracy for melanocytic lesions that are difficult to diagnose and manage,4,7,12–15 adding to the body of evidence that supports the use of confocal microscopy in clinical practice to, among other applications, avoid unnecessary biopsies.
While we recognize the limitations of this case series, we believe that our findings show that reflectance confocal microscopy can be a useful tool for evaluating lesions with equivocal features, even on dermoscopy. In all 3 cases, confocal microscopy not only helped to establish and confirm a diagnosis, but also led to changes in the final treatment decision.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Alarcón I, Carrera C, Puig S, Malvehy J. Utilidad clínica de la microscopia confocal de reflectancia en el manejo del lentigo maligno melanoma. Actas Dermosifiliogr. 2014;105:e13–e17.