Several types of cutaneous adverse reaction have been reported during anti-programmed cell death (PD)-1 antibody therapy. We describe herein rare cases of lichen planus (LP) which developed during nivolumab therapy for advanced non-small-cell lung cancer (NSCLC). Both cases involved the lower extremities, and one of which was bullous LP.
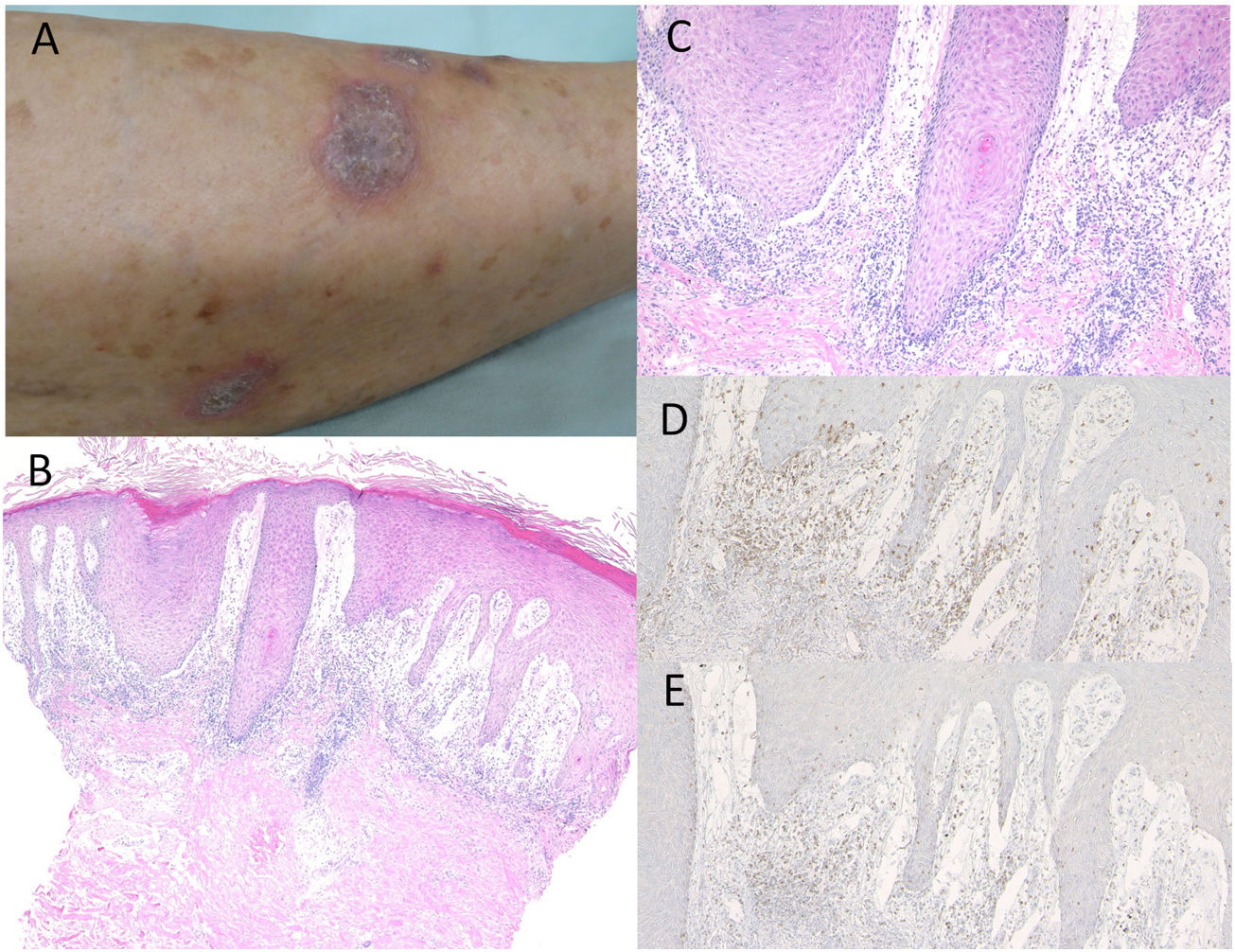
Case 1: A 74-year-old female was referred to the Department of Dermatology at Shirakawa Kosei General Hospital, complaining of skin rashes on the lower extremities. She was not taking any medications. She had been treated with nivolumab (3mg/kg) administration every two weeks for NSCLC. After the third infusion, itchy eruptions appeared on the lower extremities. Physical examination showed multiple well-defined, purple-colored keratotic plaques slightly covered with scales on the bilateral lower extremities (Fig. 1A). Histological examination showed focal hyperkeratosis with parakeratosis, focal loss of the granular layer, irregular epidermal hyperproliferation, and mild liquefaction degeneration of the basal layers of the epidermis with prominent subepidermal edema (Fig. 1B, C). Mononuclear cells, which were mainly composed of CD4- and CD8-positive T-cells, infiltrated into the upper dermis (Fig. 1D, E). Direct immunofluorescence was negative for either immunoglobulins or C3 deposition in the epidermal basement membrane. She was thereafter treated with topical corticosteroids without discontinuation of nivolumab, but with insufficient effects.
Purple-colored keratotic plaques scattered on the lower leg. (B) Histological features showing irregular epidermal proliferation with subepidermal edema and cellular infiltrates in the upper dermis. Hematoxylin–eosin stain [HE], ×100. (C) Higher magnification showing liquefaction degeneration of the basal layers of the epidermis. HE, ×200. Infiltrating cells were immunoreactive for CD4, ×200 (D) and CD8, ×200 (E).
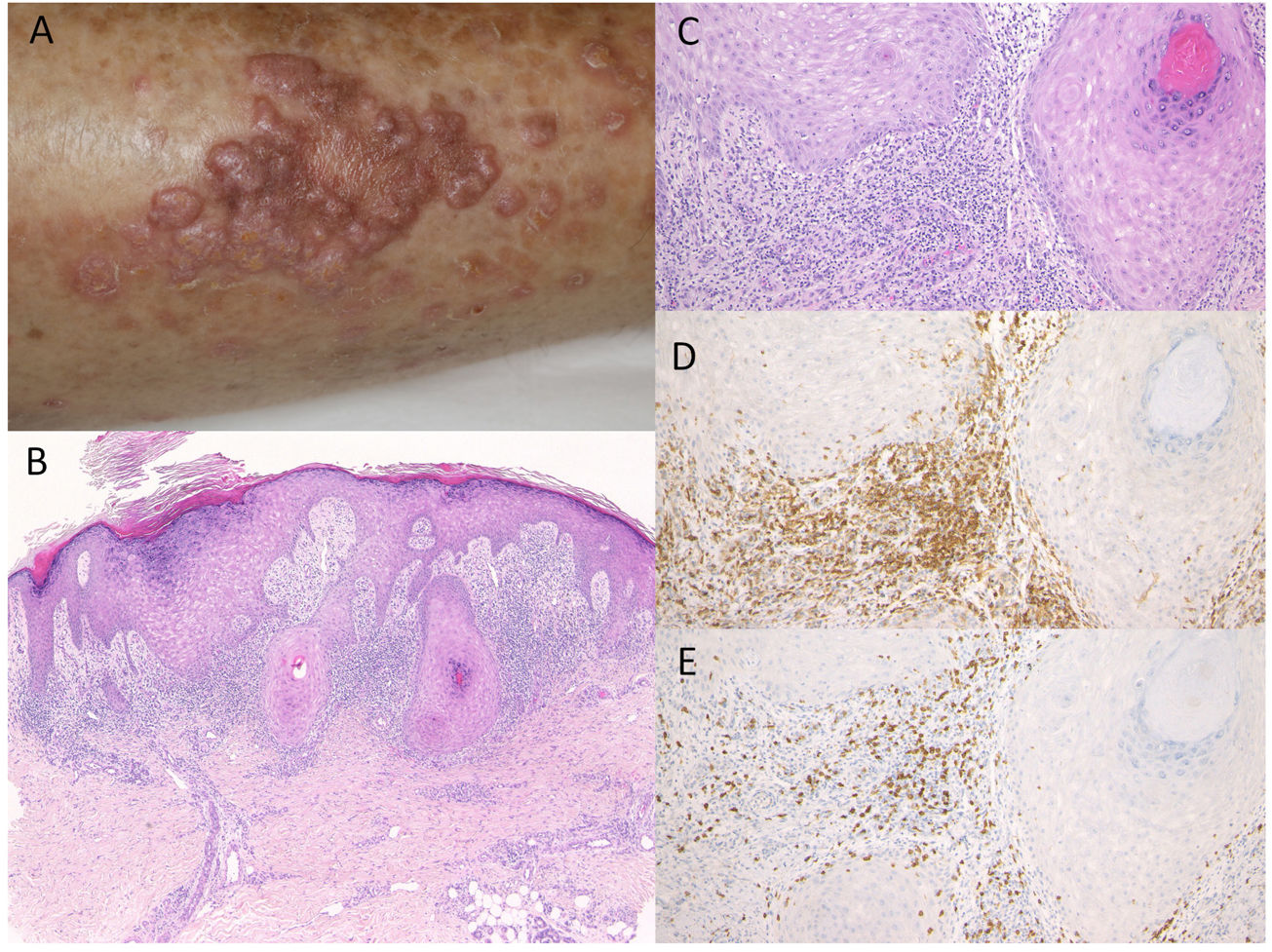
Case 2: An 81-year-old man was treated with pembrolizumab (3mg/kg) administration every three weeks for NSCLC. After the ninth infusion, he was referred to the Department of Dermatology at Fukushima Medical University Hospital, complaining of itchy skin rashes on the lower extremities. After the twelfth infusion, erythema and multiple well-defined dark-red plaques slightly covered with scales appeared on the lower thighs (Fig. 2A). Histological examination showed focal hyperkeratosis with orthokeratosis, hyperplasia of the granular layer, irregular epidermal hyperproliferation, mild liquefaction degeneration of the basal layers, and edema of dermal papillae (Fig. 2B, C). A band-like infiltrate of inflammatory cells, mainly composed of CD4- and CD8-positive T-cells, was observed in the upper dermis (Fig. 2D, E). He was treated with topical corticosteroids and oral antihistamine without discontinuation of pembrolizumab, which resulted in satisfactory effects.
(A) Multiple, well-defined dark-red erythematous plaques with scales on the lower thighs. (B, C) Histological features showing focal hyperkeratosis with orthokeratosis, hyperplasia of the granular layer, irregular epidermal hyperproliferation, mild liquefaction degeneration of the basal layers, and edema of dermal papillae. HE, (B) ×100, (C) ×200. Infiltrating cells were immunoreactive for CD4, ×200 (D) and CD8, ×200 (E).
To date, cases of exacerbation of pre-existing psoriasis or de novo induction of psoriasis/psoriasiform eruptions have been reported during nivolumab therapy; however, induction of LP is rare. There are several reports on LP or lichenoid dermatitis.1–6 In a single institution cohort study, lichenoid reactions were observed in 17% of 82 patients with metastatic melanoma who received anti-PD-1 therapy.1 Histopathologically, the degree of interface dermatitis and epidermal changes are variable. Bullous LP is commonly seen on the lower extremities. Histopathologically, biopsy from the bullous lesion is characterized by a subepidermal bulla accompanied by classical changes of LP. Bulla formation may be due to the extensive liquefaction and vacuolation of the basal layer. In Case 1, purple-colored keratotic plaques appeared on the lower legs after the initiation of nivolumab. Histopathological examination revealed irregular hyperproliferation of the epidermis with marked subepidermal edema. Wakade et al.5 reported three cases of PD-1 inhibitor (pembrolizumab) induced bullous LP-like reactions after 2–22 cycles of administration. Two of the patients had NSCLC, and the other had melanoma. Biolo et al.6 recently reported an unusual case of linear bullous LP unilaterally involved the lower extremity under treatment with nivolumab.
It has been suggested that the pathogenesis of LP is due to epidermal damage caused by autoreactive cytotoxic CD8+ T-cells, mediated by interferon-γ (IFN-γ). In murine LP models, prominent expression of PD-L1 in keratinocytes is suggested to play a protective role against cytotoxic CD8+ T-cells.7 In addition, in vitro studies showed that administration of anti-PD-1 antibodies induced increased production of IFN-γ from peripheral blood mononuclear cells of patients with oral LP.8 Komori et al. recently reported a case which developed LP focally in an irradiated area, suggesting Koebner phenomenon.9 They speculated a close relationship between anti-PD-1 therapy plus radiotherapy and the development of LP. Another recent report showed an increased mRNA expression of granzyme B and IFN-γ after nivolumab treatment.10 Inhibition of PD-1 may induce epidermal basal layer damage with prominent edema leading to bullous LP, mediated by IFN-γ and other molecules.
Conflict of interestThe authors have no conflicts of interest directly relevant to the content of this article.


![Purple-colored keratotic plaques scattered on the lower leg. (B) Histological features showing irregular epidermal proliferation with subepidermal edema and cellular infiltrates in the upper dermis. Hematoxylin–eosin stain [HE], ×100. (C) Higher magnification showing liquefaction degeneration of the basal layers of the epidermis. HE, ×200. Infiltrating cells were immunoreactive for CD4, ×200 (D) and CD8, ×200 (E). Purple-colored keratotic plaques scattered on the lower leg. (B) Histological features showing irregular epidermal proliferation with subepidermal edema and cellular infiltrates in the upper dermis. Hematoxylin–eosin stain [HE], ×100. (C) Higher magnification showing liquefaction degeneration of the basal layers of the epidermis. HE, ×200. Infiltrating cells were immunoreactive for CD4, ×200 (D) and CD8, ×200 (E).](https://static.elsevier.es/multimedia/00017310/0000011300000010/v1_202211300529/S0001731022006032/v1_202211300529/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)