Cutaneous larva migrans (LM) infection forms a serpiginous eruption caused by the migration of nematode helminths through the epidermis. The parasites are acquired when the skin comes into contact with soil contaminated by the feces of infected animals. Until now, infections have been believed to be imported from tropical and subtropical regions. Our aim was to study cases of cutaneous LM diagnosed in residents of the Spanish province of Guipúzcoa who had not recently traveled to such regions.
Material and methodsCross-sectional observational study of LM cases diagnosed in Hospital Universitario Donostia from 2011 to 2015 in patients who had not visited a region where this nematode infection is endemic. Clinical diagnoses were based on characteristic lesions. We studied the following variables: age, sex, site of lesions, date of onset of symptoms, possible source of contagion, pathologic findings, treatment, and clinical course.
ResultsWe found 4 cases, all in men (mean age, 60 years). Lesions were on the lower extremities in 3 patients and on the trunk in 1 patient. All had been in contact with soil that could have been contaminated by feces and was the most likely source of the parasite. The lesions disappeared after treatment with oral albendazole.
ConclusionsThe appearance of cases of autochthonous LM in Europe requires investigation of the culprit species, a review of the epidemiology of this infection, which was once considered imported, and the planning of public health measures to prevent it from becoming endemic.
Larva migrans cutánea (LM) es una erupción serpiginosa causada por helmintos nematodos que circulan por la epidermis. Se adquiere cuando la piel entra en contacto con tierra contaminada por heces de animales infestados por estos nematodos. Hasta ahora se consideraba como enfermedad importada de zonas tropicales y subtropicales. El objetivo fue estudiar los casos de LM diagnosticados como autóctonos por no haber salido de la provincia de Guipúzcoa recientemente.
Material y métodosEstudio observacional transversal retrospectivo de los casos diagnosticados de LM en el Hospital Universitario Donostia de 2011 a 2015, sin viaje previo a ninguna zona endémica de este cuadro. El diagnóstico fue clínico ante las lesiones características. Las variables estudiadas fueron: edad, género, localización de las lesiones, fecha de inicio de los síntomas, posible fuente de contagio, datos anatomo-patológicos, tratamiento y evolución.
ResultadosSe han recogido 4 casos, todos varones, con una media de edad de 60 años. Tres casos presentaron lesiones en las extremidades inferiores, mientras que uno lo hizo en el tronco. Todos nuestros pacientes habían estado en contacto con tierra que pudiera estar contaminada por heces, siendo este el mecanismo de transmisión más probable. Se instauró tratamiento con albendazol oral, con resolución de las lesiones.
ConclusionesLa aparición de nuevos casos de LM de origen autóctono en Europa obliga al estudio de la/s especie/s causal/es, a una revisión epidemiológica de esta infestación y a planificar qué medidas se deberían tomar para evitar que una enfermedad considerada hasta ahora como importada, se convierta en autóctona.
The term cutaneous larva migrans (LM) refers to an erythematous pruritic rash of serpiginous morphology that advances in the direction of one of its ends. It is caused by the migration of helminth nematode larvae through the epidermis,1,2 and is the most commonly imported disease of tropical origin.3,4 The diagnosis is clinical, based on observation of the characteristic skin lesions. Pathology study is unnecessary for its diagnosis; when biopsy is performed, it must be taken into account that the larva is found 1 to 2cm in front of the advancing end of the serpiginous lesion, making parasitic structures unlikely to be found in the sample.2
There are numerous species of nematodes that can cause this infestation; the main one that affects humans is Ancylostoma braziliense.5 The majority of these species are found in regions with a hot climate and, in Spain, this is therefore an imported disease.
The adult forms of the species that cause LM colonize the digestive tract of cats and dogs, from where their eggs are excreted in the feces.6 These animals are the definitive hosts,2 as the larva can complete its life cycle in them. Under optimal environmental conditions of temperature and humidity,7 eggs excreted in the feces hatch to release larvae that mature until they reach the filariform (infective) stage. On reaching this phase, the larvae can penetrate the skin to infect a new animal and thus complete their life cycle, or else they can enter the skin of a human being. On penetration, the larva provokes the appearance of a small, pruritic erythematous papule or vesicle, and opens a path through the epidermis, advancing at a rate of approximately 2 to 3cm a day1; this is the cause of the characteristics lesions, which are serpiginous, erythematous, and intensely pruritic. Several serpiginous tracts can often be seen simultaneously in a single patient.8 As the larvae advance, the initial section of the lesion becomes dry and crusted. Lack of the enzyme necessary to cross the basement membrane of human skin (collagenase)9 means that the larvae cannot reach the blood or lymph vessels, and they therefore migrate through the epidermis for an indeterminate time until they die without being able to complete their life cycle. Even so, a case of visceral LM due to Ancylostoma caninum has been published.10
The clinical course is towards spontaneous resolution of the lesions over a period of 1 to 2 months due to the immune response that is triggered.2 Cases of lesions that have persisted for 2 years have been reported.11 Possible complications, such as bacterial superinfection and allergic reactions, and the intense pruritus make treatment recommendable.
The objective of this study was to review cases of LM with no epidemiologic history of recent travel to endemic regions; it thus refers only to autochthonous cases.
Material and MethodsThis was a retrospective observational study of patients diagnosed with LM in the dermatology department of Hospital Universitario Donostia between August 2011 and June 2015. All patients lived in Gipuzkoa, Spain, and had no history of recent travel outside this province.
Variables analyzed included age, sex, date of onset of the condition, nature of possible contact with soil contaminated by larvae, site of the lesions on the body, findings on biopsy (if performed), and climatic conditions during the previous months that could have favored the onset of the disease.
ResultsFour patients, all men, were studied. The mean age was 60 years (range, 39-79 years). They denied recent foreign travel, but they did report recent contact with soil possibly contaminated by animal feces in the rural environment. Table 1 summarizes the main clinical and epidemiologic characteristics of each patient and the possible context in which contact with the larvae occurred.
Clinical and Epidemiological Characteristics of the Patients in the Series.
| Age | Site Affected | Onset | Possible Contact | Biopsy | Treatment | Clinical Course | |
|---|---|---|---|---|---|---|---|
| 1 | 60 | Dorsum of the foot | August 2011 | Trekking using sandals | No | Oral albendazole | Good initial clinical course Subsequent reinfestation Resolved by changing footwear |
| 2 | 63 | Trunk, arms | September 2013 | Gardening without a shirt | Yes. Perivascular dermatitis | Oral albendazole | Resolution after treatment |
| 3 | 79 | Lower leg, dorsum of the foot | August 2014 | Gardening | Yes. Perivascular dermatitis | Oral albendazole | Resolution after treatment |
| 4 | 39 | Lower leg | June 2015 | Trekking | No | Oral albendazole | Resolution after treatment |
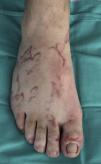
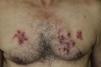
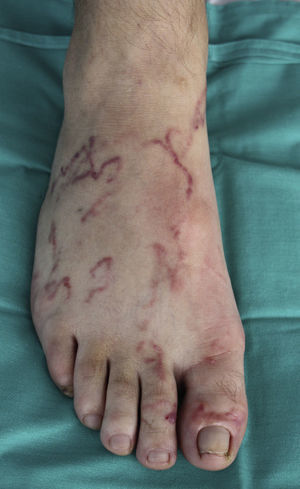
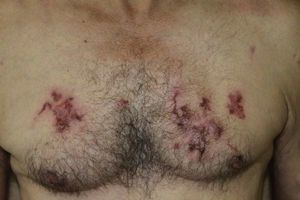
The site of the skin lesions was on the lower limbs in 3 cases (Fig. 1) and on the trunk in 1 (Fig. 2). This last patient described being stripped to the waist while working in his garden and repeatedly entering into contact with soil that could be contaminated.
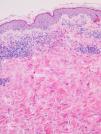
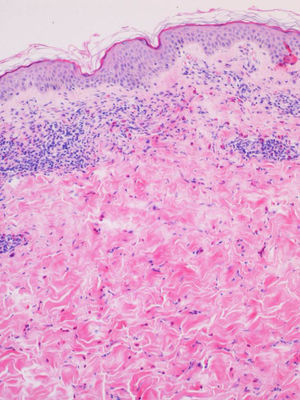
Biopsy and blood tests were performed in 2 patients, with similar findings in both cases. Histopathology revealed a superficial and deep perivascular dermatitis with a predominance of eosinophils (Fig. 3). The blood tests showed peripheral eosinophilia of 13% and 17%, respectively; other parameters were within normal limits.
The diagnosis was made clinically, based on the characteristic skin lesions and the epidemiologic history of contact with soil possibly contaminated by animal feces.
The treatment prescribed in all 4 cases was albendazole 400mg a day for 5 days, with a good clinical response in 3 of them. The fourth patient presented recurrence despite a good initial response to the antiparasitic treatment. We found that his sandals were dirty with soil. After changing the footwear and restarting systemic treatment, the lesions disappeared.
DiscussionBetween 2011 and 2015, 4 cases of LM were diagnosed in our center in patients who did not report recent travel outside the province of Gipuzkoa; these were therefore autochthonous cases of LM. Both the medical history and the sites of the lesions indicated occupational exposure, as all 4 patients had been in contact with soil probably contaminated by parasite-infected animal feces.
LM is observed predominantly in travelers arriving from hot and humid regions, particularly Sub-Saharan Africa, Latin America, the Caribbean, and Asia.2 In recent years, a total of 15 autochthonous cases have been detected in western European countries: Germany,12 England,13–16 France,17–19 Italy,20 Spain,2,21 and Serbia.22 The main clinical and epidemiological characteristics of all cases of autochthonous LM in Europe are shown in Table 2. We bring 4 new cases (21% of the total) to add to those already reported in our continent. A total of 6 cases (32%) have been reported in Spain, making this the European country with the highest incidence of autochthonous LM in the last decade.
Clinical and Epidemiological Characteristics of Cases of Larva Migrans Arising in Europe With no History of Travel.
| Author | Country | No. of Cases | Age, y | Site Affected | Period of the Year | Related Activity | Clothing | Time Without Travelling to the Tropics | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Beattie et al. | United Kingdom (Scotland) | 1 | – | Left buttock and flank | – | Trekking | Short trousers | 3 y | Oral ivermectin |
| Diba et al. | United Kingdom | 1 | 50 | Left arm Abdomen | October | Paint-ball | Paint-ball clothing in contact with dog | 3 y | Oral albendazole |
| Roest et al. | United Kingdom | 1 | 47 | Right buttock | September | Sitting on the beach | – | – | Oral albendazole |
| Patterson et al. | United Kingdom | 1 | 10 | Left foot | Winter | Contact with dog feces | Barefoot | Never | Topical thiabendazole |
| Klose et al. | Germany | 3 | 7, 38, and 44 y | Feet (2) and right hand (1) | August | Banks of a river and of a lake | – | Never | Topical thiabendazole |
| Ropars et al. | France (Brittany) | 1 | 69 | Left flank | – | Hiking around a lake | – | – | Oral ivermectin |
| Tamminga et al. | France (Brittany) | 1 | 40 | Left foot | September | Walking on a beach Camping | – | Never | Oral ivermectin |
| Zimmermann et al. | France | 1 | – | Right leg | – | Flooding of patient's house | – | Topical and oral thiabendazole | |
| Akkouche et al. | Italy | 1 | 42 | Upper limbs | – | Veterinary paramedic Contact with stagnant water | Gloves not used when working with animals | Never | Oral albendazole |
| Santiago et al. | Spain (Pyrenees) | 1 | 31 | Buttocks | – | Riverbank | Bathing suit | – | Oral albendazole |
| Sábat et al. | Spain | 1 | 22 | Thumb | – | Contact with dogs | – | – | Thiabendazole oral |
| Tomović et al. | Serbia | 2 | 63 and 70 | Trunk (1) Back (2) | August (1) June (2) | Fall in sand (1) Repairing a car | Not wearing a shirt (2) | Never | Oral albendazole (1) Oral ivermectin (2) |
These findings would suggest that the incidence of autochthonous cases of this condition is likely to increase in the future. The presence of skin lesions compatible with LM must therefore lead to inclusion of this disease in the differential diagnosis even if the patient reports no recent travel outside Europe.
The etiologic agents of LM are numerous. The possible hosts and the geographic distribution of each of them are listed in Table 3.2,6,7
Hosts and Geographic Distribution of the Causative Species of Cutaneous Larva Migrans.
| Host | Distribution | |
|---|---|---|
| Ancylostoma braziliense | Dogs Cats | Tropical and subtropical regions |
| Ancylostoma caninum | Dogs Occasionally cats | Worldwide distribution |
| Ancylostoma tubaeforme | Cats Other felines | Worldwide distribution |
| Ancylostoma ceylanicum | Canines Felines | Africa, Australia, and Asia |
| Uncinaria stenocephala | Dogs Occasionally cats | Temperate regions |
| Bunostomun phlebotomum | Cattle | Temperate regions |
The intestinal nematodes Ancylostoma spp. and Uncinaria spp. are the most prevalent groups of parasites in household pets worldwide; pets are among the most important sources of spread of the disease and of risk to human and animal health.6 This is due above all to their life cycle, their route of transmission, and the large number of eggs produced by each female. In animals, the skin lesions are typically interdigital and are hidden by the hair.6 After penetrating the skin, the parasite reaches the bloodstream and migrates to the lungs, from where it is swallowed and reaches the intestine, the site where it matures to the adult phase. In the intestine, the parasite feeds on the animal's blood, producing anemia, blood-stained diarrhea, and even death in young animals.7 The females produce enormous numbers of eggs (around 20000 eggs a day), which are excreted with the host's feces. In the environment, under suitable climatic conditions of warmth and humidity (optimal temperatures vary between 25°C and 30°C; moist, sandy, shady, and well-oxygenated soil), the eggs hatch to produce larvae that pass through 2 stages to reach the L3 stage. The L3 larva is filariform, measures about 660μm in length and 2μm in diameter, and is infective for dogs and humans, as it is able to penetrate the skin.7
In humans, the infection is typically acquired on walking barefoot or sitting on soil contaminated with L3 larvae derived from infected animal feces; LM lesions are therefore usually observed on the lower limbs and buttocks.2,5 Lesions can also appear in other regions, such as the trunk or scalp.
The differential diagnosis should include larva currens, caused by Strongyloides stercoralis, in tropical and subtropical regions.23 Clinically, these diseases are differentiated mainly by the greater speed of migration through the epidermis; the lesions in larva currens progress at 5 to 15cm per hour2 and the path is relatively straight, whereas LM advances at 2 to 3cm a day in a more serpiginous path. Humans can be the definitive host of Strongyloides stercoralis, as this parasite has the potential to cross the basement membrane of the skin to reach blood and lymph vessels, thus completing its life cycle. Migration through the human body can provoke iron-deficiency anemia, gastrointestinal symptoms, and Loeffler syndrome. This syndrome is characterized by peripheral eosinophilia and migratory pulmonary infiltrates caused by passage of the larvae through the lungs. Cases of Loeffler syndrome caused by LM have also been reported.24
Climate change is altering the ecology of a number of helminth species with zoonotic potential. The increase in temperatures is particularly important for those parasite species that complete part of their life cycle in the soil, 1 of the main routes of transmission of this zoonosis. All the months in which a diagnosis of LM was made at our center had similar meteorological characteristics. In August 2011, air from the Sahara reached the north coast of Spain, producing unusually high temperatures. In addition, the climatic instability associated with that low pressure produced stormy showers on several days, with rain recorded on 14 days. In September 2013, it rained on 15 days, with heavy storms on 4 of them. And there were 2 clear episodes of entry of tropical air at the beginning and end of the month, with maximum temperatures of 36°C in the west of Gipuzkoa. August 2014 had a particularly high rainfall, with rain present on 19 days. The number of hours of sun was below average (by about 20%), with only 1 cloudless day. In June 2015, it rained for a total of 16 days, mainly in the east of Gipuzkoa. It was a very warm month, with maximum temperatures of 38°C on the Gipuzkoan coast.
It is likely that the increasing temperatures and high humidity in the north of the Iberian Peninsula, and the low levels of solar radiation compared with other areas of Spain, will create an ideal environment for eggs of parasites that cause LM to hatch in our soil and for the larvae to develop, especially in the warmer summer months.
Although current veterinary guidelines recommend parasite prevention for dogs and cats every 3 months, these periodic controls may not be carried out in rural environments or animals may become reinfested within this period, or feral animals (especially cats) may be involved. This will facilitate the spread of the parasite and the appearance of new cases.
Several systemic treatment options exist. Oral treatment can be given as a single dose of albendazole 400mg by mouth. A 3- or 5-day course of treatment with doses of 400 or 800mg a day is more effective,25 with a complete response in 99% of cases. Thiabendazole is also effective at doses of 1.25-2.5g a day,26 but this drug is not as well tolerated as ivermectin or albendazole, as it can cause nausea, vomiting, and headache. A single dose of 12mg of ivermectin is effective and is well tolerated.27
If topical therapy is used, the application of thiabendazole at concentrations of 15% or 6.25% twice a day achieves resolution of the disease with 2 to 8 days of treatment.28 Cryotherapy is not recommended as it is highly traumatic and the larvae are found some centimeters in front of the advancing end of the lesion.8,26 Cure has also been observed after skin biopsy.2 Clinical resolution is usually seen a week after starting treatment. Preventive measures must be adopted to prevent greater spread of this disease. Veterinary controls to deparasitize dogs and cats should be performed every 3 months, with special insistence in rural areas. Another factor to be taken into account is the involvement of wild and feral animals, such as foxes and cats, which can also spread these larvae and are more difficult to control. Health staff should be trained to recognize this disease so that it can be quickly identified.2 Patients will then receive early treatment and epidemiologic information can be gathered about possible sources of contact to prevent further contagion. To this end, it would be very interesting to know the species of larvae implicated in each case in order to be able to direct treatment to the parasitized animals or against the mechanism of transmission. It is important to insist on barrier methods, such as the use of closed footwear and appropriate clothing to avoid contact with soil contaminated by animal feces. Cleaning or changing footwear must be included as part of the treatment of this disease; this is important to prevent reinfestation in the same individual and contamination of urban areas. The possibility of short-term dissemination in the urban setting must also be taken into account, as contaminated footwear and other fomites can infest carpets,29 where the eggs can live for several days. Town parks, which can be moist shady areas, could be a suitable setting for the hatching of eggs excreted in the feces of parasitized household pets. Identification of the parasite in the 4 cases reported would provide data on the prevalence, life cycle, and hosts of these larvae, and this information could be used to prevent further spread of the species that cause this condition in our setting.
ConclusionLM is an easily recognized condition that is usually associated with a history of travel to areas in which this dermatosis is endemic. However, autochthonous cases occur in Europe, and our series is the largest published to date. We should expect an increase in the incidence of this disease in both rural and urban areas in the warmer months (June through September) and in moist environments. The rapid identification of this dermatosis, the initiation of an effective treatment, knowledge of the sources of contagion, and preventive measures can be effective in preventing greater spread of LM of autochthonous origin.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this research.
Confidentiality of dataThe authors declare that they followed their hospital's regulations regarding the publication of patient information.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Panés-Rodríguez A, Piera-Tuneu L, López-Pestaña A, Ormaetxea-Pérez N, Gutiérrez-Támara P, Ibarbia-Oruezabal S, et al. Larva migrans cutánea de origen autóctono en Guipúzcoa. Actas Dermosifiliogr. 2016;107:407–413.