The reproducibility of patch test results is of critical importance due to their use as a diagnostic tool in allergic contact dermatitis.1,2 This study attempted to validate the reliability of the results obtained with allergEAZE® (AE), a new standardized brand of syringe-administered allergens for patch tests. This approach is used as a method of diagnosing contact dermatitis; the diagnosis being contingent on the achievement of reproducible results as compared to other commonly used conventional systems.
The study included patients who were suspected to have contact dermatitis. They underwent duplicate testing with 5 different allergens from the Spanish standard patch test series. Nickel sulphate hexahydrate, potassium dichromate and 4-phenylenediamine base were the same for all the researchers, and the tests revealed a high frequency of positive reactions. The other two allergens were random and were less frequently positive. We tested the allergens of the new brand on one side of the back. The same allergens were used, but from different brands, employing those usually chosen by each researcher (True Test® (TT), Trolab® (TRO), Martí Tor® (MT) or Chemotechnique® (CH)), and were tested symmetrically on the opposite side of the back. In patients tested with petroleum jelly allergens, the concentrations recommended by the Spanish Research Group on Dermatitis and Cutaneous Allergy (GEIDAC) were used, and were applied indistinctively from the patch test with Finn Chambers® on Scanpor® (SmartPractice) and Curatest® (lohmann-rauscher). Standard patch test recommendations were followed during the course of the study. Any degree of positivity on D4 was considered a positive result. Any reactions that showed signs of irritation or were doubtful were included in the group of negative results. Positive concordance was defined as a positive reaction to the same allergen by two different methods. The formula used to calculate the percentage of positive concordance was: 100×[number of positives to both tests: AE+other+]/[sum of all the positives: (AE+other+) plus (only AE+) plus (only other+)].
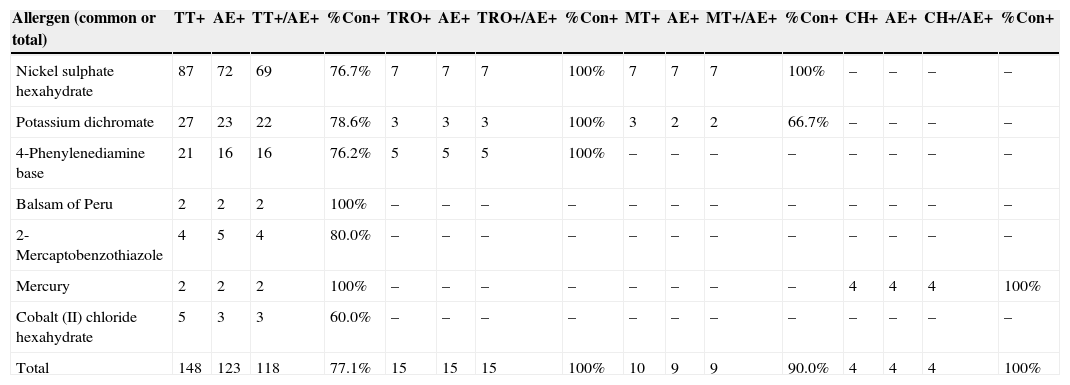
The analysis of the results is summarized in Table 1.
Positive resultsa obtained and positive concordance rates among different methods.
| Allergen (common or total) | TT+ | AE+ | TT+/AE+ | %Con+ | TRO+ | AE+ | TRO+/AE+ | %Con+ | MT+ | AE+ | MT+/AE+ | %Con+ | CH+ | AE+ | CH+/AE+ | %Con+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nickel sulphate hexahydrate | 87 | 72 | 69 | 76.7% | 7 | 7 | 7 | 100% | 7 | 7 | 7 | 100% | – | – | – | – |
| Potassium dichromate | 27 | 23 | 22 | 78.6% | 3 | 3 | 3 | 100% | 3 | 2 | 2 | 66.7% | – | – | – | – |
| 4-Phenylenediamine base | 21 | 16 | 16 | 76.2% | 5 | 5 | 5 | 100% | – | – | – | – | – | – | – | – |
| Balsam of Peru | 2 | 2 | 2 | 100% | – | – | – | – | – | – | – | – | – | – | – | – |
| 2-Mercaptobenzothiazole | 4 | 5 | 4 | 80.0% | – | – | – | – | – | – | – | – | – | – | – | – |
| Mercury | 2 | 2 | 2 | 100% | – | – | – | – | – | – | – | – | 4 | 4 | 4 | 100% |
| Cobalt (II) chloride hexahydrate | 5 | 3 | 3 | 60.0% | – | – | – | – | – | – | – | – | – | – | – | – |
| Total | 148 | 123 | 118 | 77.1% | 15 | 15 | 15 | 100% | 10 | 9 | 9 | 90.0% | 4 | 4 | 4 | 100% |
True Test® (TT), allergEAZE® (AE), Trolab® (TRO), Martí Tor® (MT) and Chemotechnique® (CH).
%Con+: positive concordance rates.
In total, 289 patients from 18 different centers were recruited and tested by researchers from the GEIDAC. A total of 2890 patch tests were performed, and 328 positive results were obtained (11.35%). After an analysis of the results, the general positive concordance rates between the other brands (TT, TRO, MT and CH) and AE were 77.1%, 100%, 90% and 100%, respectively. These results have been obtained without taking the degree of positivity into account.
Patch test diagnosis is a biological assay with a high degree of inherent variability. Factors that affect the results include the concentration, dosage and the use of an adequate vehicle, as well as variations in absorption and reactivity. There may also be differences between testing two identical allergens on different parts of the back. For example, the positive concordance rate between TT and CH varies between 63% and 78% in various studies.1 In a study comparing 20 allergens using AE and CH on 21 patients, the authors found the largest differences in reactions to quaternium-15, nickel and cobalt. They concluded that, although both series are valid for the diagnosis of contact dermatitis, additional studies with a larger population would be required to determine the relative effectiveness of both methods.2
Our study proves that the use of AE allergens for patch tests provides the same results as other conventional brands in more than 75% of allergen cases, with a high positive concordance rate among the tested methods for nickel sulphate hexahydrate, potassium dichromate, 4-phenylenediamine base, balsam of Peru, 2-mercaptobenzothiazole, mercury and cobalt (II) chloride hexahydrate, although there may be differences in the of positivity.
Conflicts of interestNo one involved with this study has accepted travel funds or research contributions of any kind. L.C. Sáez-Martín is an employee of Marti Tor Impomedic, S.L.
The allergEAZE® allergens were provided by SmartPractice®.