Actinic keratoses (AK) are common cutaneous lesions located in skin areas chronically exposed to UV radiation, with the potential of progressing into invasive squamous cell carcinoma (SCC). Since it is not possible to predict which AK lesions will become SCC or when will that happen, treating all AK is generally recommended. There is a wide range of therapies available for AK and new drug approvals have joined the therapeutic armamentarium in recent years. These changes in the available treatments for AK require a review of the scientific evidence available and the current status of AK diagnosis and management in Spain.
Las queratosis actínicas (QA) son lesiones cutáneas frecuentes que surgen en la piel crónicamente expuesta a los rayos ultravioleta y que potencialmente pueden progresar a un carcinoma epidermoide cutáneo (CEC) infiltrante. Debido a que no se puede predecir qué lesiones progresarán a CEC ni cuándo, generalmente se recomienda el tratamiento de todas las QA. Existe un amplio abanico de tratamientos disponibles para la QA a los que se les han unido nuevas aprobaciones en los últimos años. Estos cambios en los tratamientos disponibles para la QA hacen necesaria la revisión de la evidencia científica y del estado actual del diagnóstico y manejo de la QA en España.
Actinic keratosis (AK) is a common reason for dermatological consultation in Spain, accounting for up to 6% of visits.1 Most authors agree in considering AK as a form of squamous cell carcinoma in situ of the skin.2
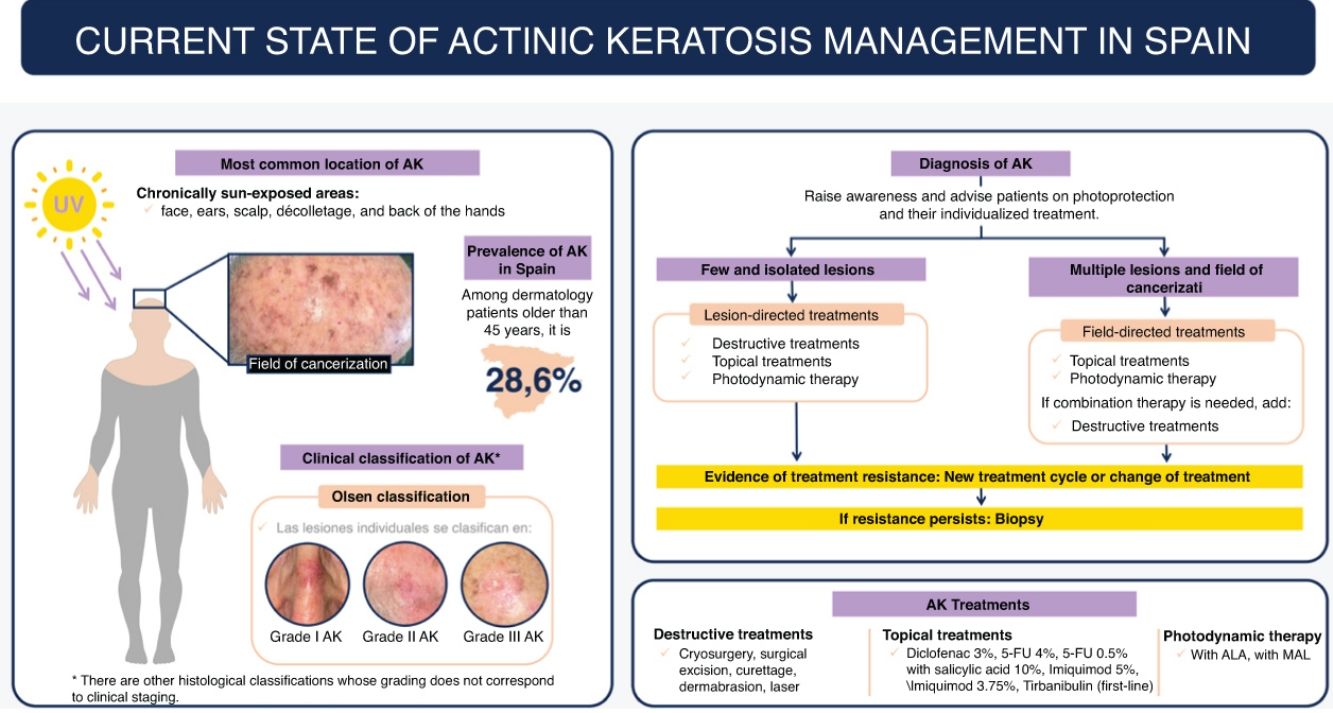
The global prevalence of AKs is 14%, according to a meta-analysis of 60 observational studies, and its incidence rate is estimated at 1.9 per 100,000 person-years.3 In Spain, the prevalence of AK in patients aged 45 or older attending dermatological consultations is 28.6%, which increases with age (45.2%, 71–80 years).4
The main cause of AKs is chronic exposure to ultraviolet (UV) radiation, especially UV-B.5 This type of radiation induces mutations in the tumor suppressor gene p53, crucial for inducing apoptosis of damaged cells, leading to uncontrolled proliferation of these cells and the appearance of AK.2 In addition, UV radiation induces mutations in other genes, such as H-Ras (regulator of cell proliferation), or the tumor suppressor p16, contributing to the development of AK.2,6,7 On the other hand, infections with the human papillomavirus could also be related to AK.2,8
Although few AKs progress into invasive cutaneous squamous cell carcinoma (cSCC) (0.07–0.6% within the first year and 2.6% in 4 years),9,10 it is important to note that approximately 60–65% of infiltrating cSCCs arise from AKs.9,10 Therefore, AKs are considered a risk marker for cSCC and the most important independent risk factor for its development.9,10 Although not all AKs progress to cSCC, there are no reliable markers to identify which ones will. Of note, some AKs may regress spontaneously or persist as AKs without progressing to dermal infiltration.11 The spontaneous regression rate varies between 15% and 63% per year for individual lesions.10,11 However, AKs that involute may reappear, with an estimated recurrence rate of 15–53% per year.11
The latest recommendations for the evaluation and treatment of patients with AK in Spain were published in 2017.12 Since then, there have been relevant changes both in its diagnosis and staging and in its management, so the objective of this publication is to offer an update on the current state of AK in our country.
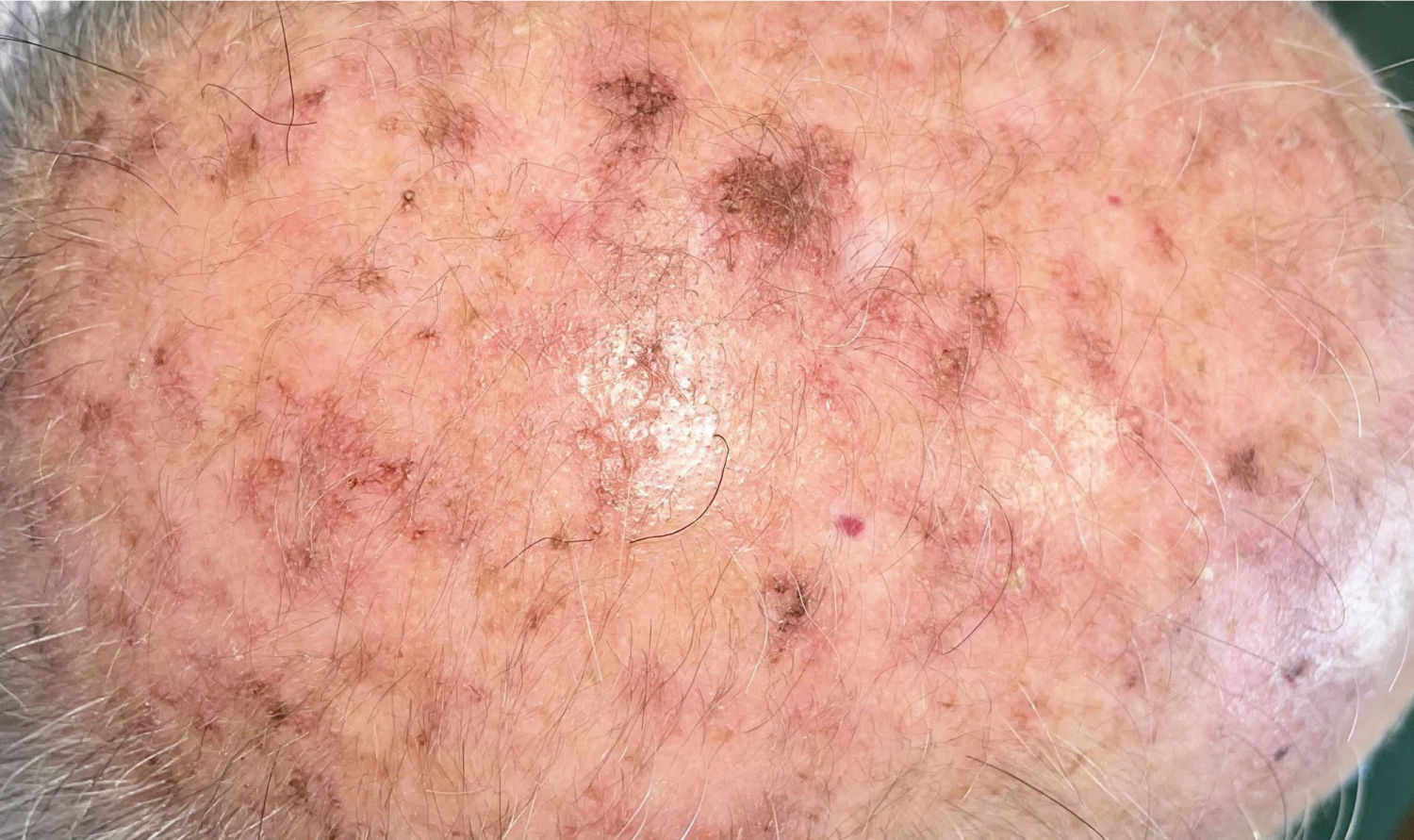
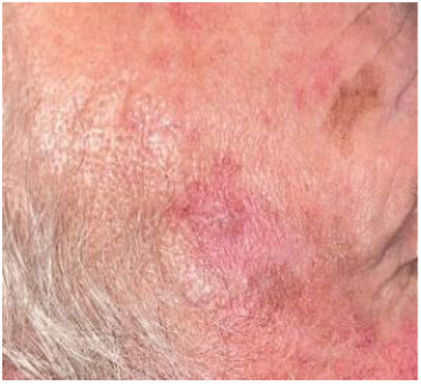
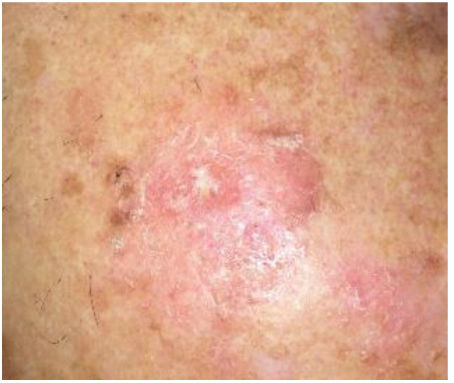
DiagnosisClinical diagnosisAKs usually present as non-infiltrated macules, papules, or plaques, erythematous or skin-colored, with a rough texture and a size of a few mm to 2–3cm in diameter.12 The most frequent locations are areas chronically exposed to the sun: face, ears, scalp (in people with alopecia), decolletage, and dorsum of the hands. Depending on the intensity or predominance of certain signs, some clinical variants can be differentiated, such as the hypertrophic or pigmented form.13,14
Clinical evaluation has a sensitivity rate of 98% and a specificity rate of 62% for detecting AK.15
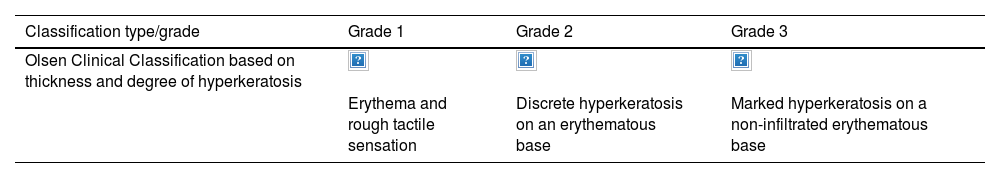
Individual AK lesions can be clinically classified according to the Olsen scale into grades 1–3 based on thickness and hyperkeratosis (Table 1).16 A 2022 study observed that the risk of progression to infiltrating cSCC in a treated area was 3.7% at 5 years, being higher in hyperkeratotic AKs (Olsen grade 3) than in grade 1/2 AKs (20.9% vs 2.4%).17
Clinical and histological classifications of AK.
| Classification type/grade | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Olsen Clinical Classification based on thickness and degree of hyperkeratosis | |||
| Erythema and rough tactile sensation | Discrete hyperkeratosis on an erythematous base | Marked hyperkeratosis on a non-infiltrated erythematous base |
| AKI | AKII (PROII) | AKIII (PROII) | |
|---|---|---|---|
| Röwert-Huber Histological Classification based on intraepidermal extension of atypical keratinocytes | |||
| Atypical keratinocytes limited to the lower third of the epidermis | Atypical keratinocytes in the lower two-thirds of the epidermis | Atypical keratinocytes throughout the epidermis |
| Crowding (PRO I) | Budding (PRO II) | Pappillary sprouting (PRO III) | |
|---|---|---|---|
| PRO Histological Classification based on basal growth pattern of atypical keratinocytes | |||
| Atypical keratinocytes are concentrated in the basal layer of the epidermis | Small buds of atypical keratinocytes from the basal epidermis protrude slightly into the papillary dermis | There is a pronounced basal growth pattern with clusters of atypical keratinocytes forming elongated papillae that protrude into the dermis |
AK: actinic keratosis.
* The grading of the 3 classifications does not correlate among them.
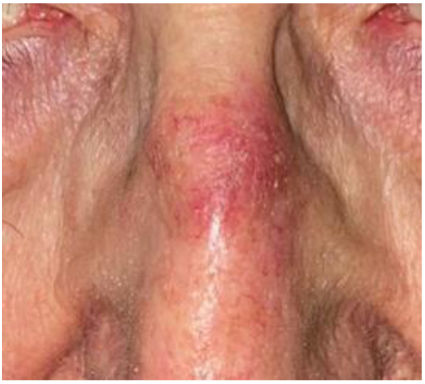
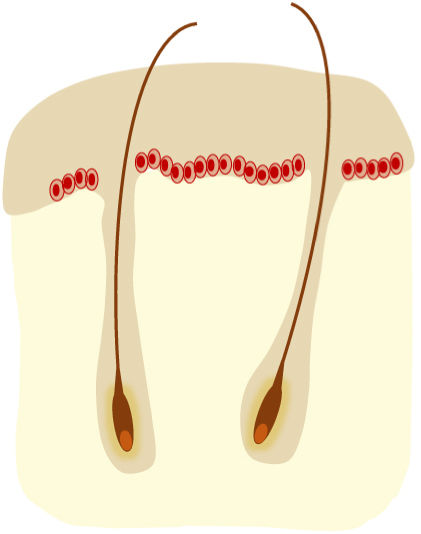
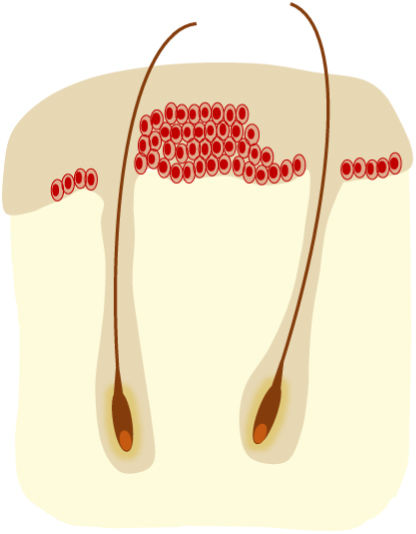
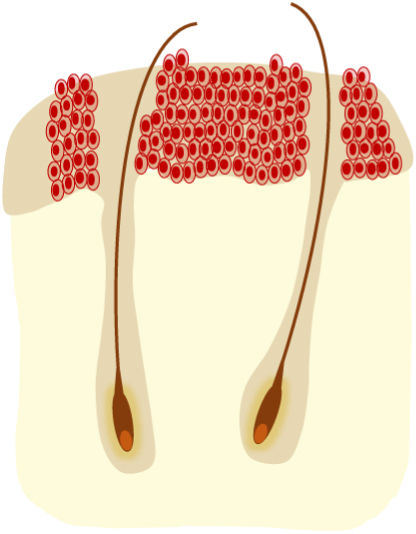
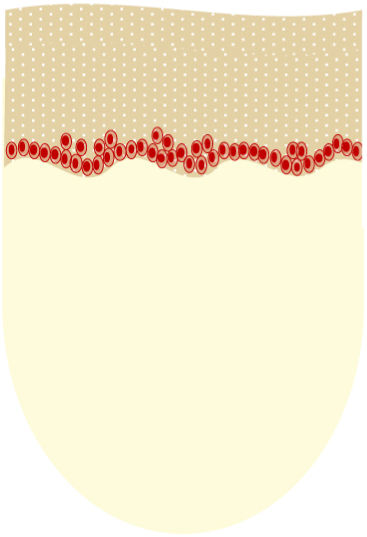
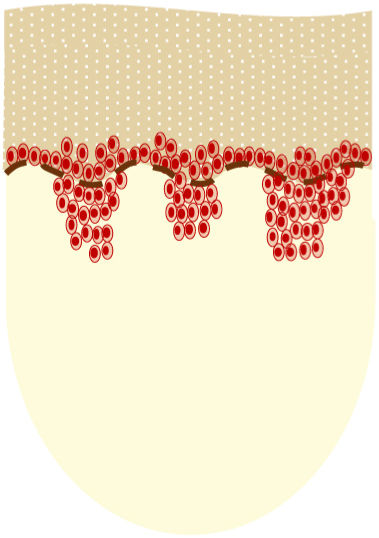
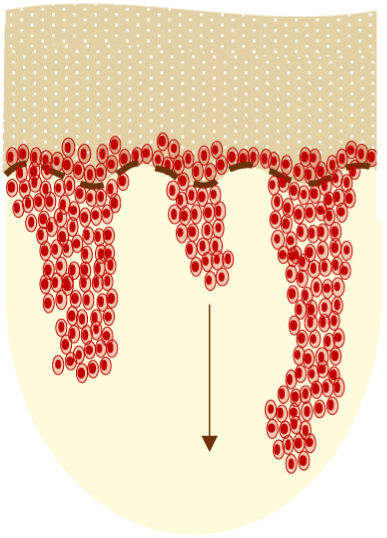
AKs are usually multiple, however. Therefore, the concept of “field cancerization” refers to the area of skin chronically damaged by the sun, characterized by, at least, 2 of the following signs: telangiectasias, atrophy, pigmentary changes, and sandpaper-like appearance.18 In this area, clinically visible AKs can be found (although there is no agreement on whether their presence is essential or not), subclinical AKs (diagnosable microscopically), clusters of keratinocytes with genetic alterations (detectable by molecular studies), and areas of normal skin (Fig. 1). Of note, histological and molecular changes common to AK and squamous cell carcinoma have been described in apparently normal skin,18 suggesting a probable benefit of treating not only individual AKs but also the field cancerization.
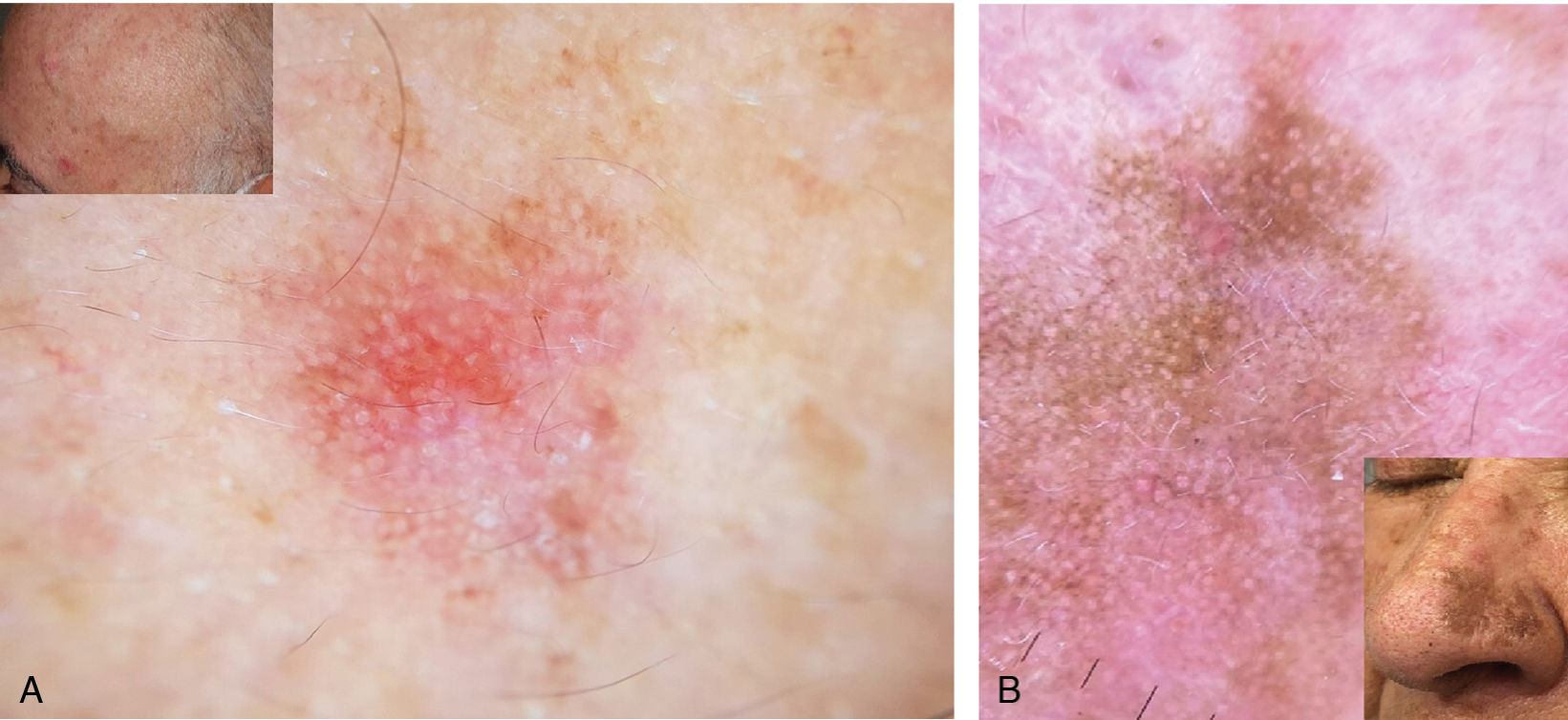
Imaging modalitiesDermoscopyDermoscopy is widely used to diagnose AK,19,20 with sensitivity and specificity rates of 98% and 95%, respectively (Fig. 2).21–23 Common dermoscopic features of non-pigmented AKs include rosettes, erythema, pink pseudoreticulum, yellowish follicular openings, scales, and fine vessels. In 95% of cases, the combination of these features produces a characteristic strawberry pattern.21
Reflectance confocal microscopyReflectance confocal microscopy (RCM) has a sensitivity and specificity in the diagnosis of AK of 80% and 96%, respectively.12,24 Its low penetration, of 200–300μm, particularly in hyperkeratotic lesions, is a limitation.24 The main observable features are the presence of superficial alteration with scales and parakeratosis, and the structural disorder and cellular pleomorphism of the spinous and granular layers with a disruption of the honeycomb pattern characteristic of these layers.24,25
Optical coherence tomographyOptical coherence tomography (OCT) is useful for identifying AKs, as it can penetrate up to the dermoepidermal junction, with a sensitivity rate of 86% and a specificity rate of 83%.26,27 Some of the described features include thinning of the epidermis with destruction of the dermoepidermal layer, white striae, dots and gray areas corresponding to hyperkeratosis, and a dark band in the stratum corneum.28,29
Histopathological diagnosisHistological confirmation is necessary if the clinical presentation is atypical, to rule out infiltrating cSCC, or in AKs that do not respond to treatment.2 Microscopically, AKs are intraepidermal proliferations of atypical keratinocytes (with enlarged, pleomorphic, hyperchromatic nuclei and increased mitotic activity), indistinguishable from the keratinocytes of infiltrating cSCCs, which implies that AKs are considered squamous cell carcinomas in situ.
According to the Röwert-Huber et al. histological classification,30,31 3 grades (AK I–III) can be distinguished based on the intraepidermal extension of atypical keratinocytes (Table 1). The Olsen classification does not correlate well with the Röwert-Huber classification, so no conclusions can be drawn about the histological grade from the clinical appearance of AKs.32 A histological study33 observed that AK I was the most frequently found lesion at the base or edge of an infiltrating cSCC, describing a progression pathway where an AK I can evolve directly to cSCC.
Another histological classification for AKs includes the evaluation of the basal growth pattern of atypical keratinocytes (Table 1),34 which has been related to the risk of progression to cSCC. One study35 observed that AKs with a pronounced basal growth pattern (PRO III) appeared more frequently near infiltrating cSCCs.
Since there is no correlation between the histological classification AK I–III and the basal growth pattern PRO I–III, the histological classification should consider both ascending and descending growth patterns.34
ScalesAKASI and AK-FASIn recent years, a series of scales have been described that allow the evaluation of the entire area affected by AK and not only individual lesions. The Actinic Keratosis Area and Severity Index (AKASI)36 was developed to assess the severity of AKs on the head. For its calculation, the head is divided into 4 areas, assigning a weighting according to their relative size. In each zone, the percentage of the affected area, the distribution of AKs, erythema, and thickness are scored. The AKASI score ranges from 0 (no AK) to 18 (most severe grade).
The Actinic Keratosis Field Assessment Scale (AK-FAS)37 evaluates the severity of the field cancerization. It considers the extent of the area affected by AK, the degree of hyperkeratosis, and sun damage, and distinguishes 4 severity grades.
AKQoLAKs affect the patients’ quality of life due to their location in visible areas, the fear of progression,38,39 and the local effects of treatments.40
The Actinic Keratosis Quality of Life (AKQoL) questionnaire41 evaluates the quality of life of patients with AK and is validated in Spain.42 It includes 3 domains: “Function,” “Emotions,” and “Control over life in general,” with scores between 0 and 27, where a higher score indicates a greater deterioration in quality of life.
Although the AKASI and AK-FAS scales and the AKQoL questionnaire are used in various studies or clinical trials, their application in clinical practice is still limited.
PreventionPhotoprotectionThe treatment of AKs should be complemented with sun protection measures.12 Photoprotection helps reduce the incidence rate of new lesions and slow the progression of existing ones.43,44 Physical filters (mineral substances such as zinc oxide, which reflect solar radiation), chemical filters (molecules that absorb UV radiation), or biological filters: endonucleases and photolyases (useful in the prevention and reversal of photodamage) are available on the market.45 Of note, the evidence on the use of photolyases for the prevention of AK is limited and comes from studies mostly conducted in combination with sunscreens. These studies generally include small samples and variable follow-up periods, making it difficult to draw any definitive conclusions on their efficacy profile as a single therapy. Thus, while photolyases may have a complementary role in protection vs photodamage, their optimal effect seems to depend on their use together with other traditional photoprotective agents.45–49
Oral chemoprophylaxisMainly in immunosuppressed individuals, chemoprophylaxis with oral retinoids (acitretin 10–25mg/day according to tolerance) has been considered an effective strategy for preventing AK and cSCC.44,45 However, the evidence supporting the efficacy profile of oral retinoids is limited, and most available studies are based on small samples. Of note, the preventive effect disappears upon discontinuation of treatment. In addition, tolerance is not good, so they are recommended as intermittent treatments.50–52
Oral nicotinamide (500mg/12h) has been shown to reduce keratinocyte carcinomas by 23% vs placebo, but AKs only by 13% at the 12-month follow-up. In kidney transplant recipients (KTRs), it proved to be safe and effective, although the differences were not statistically significant.53,54
TreatmentWhen to treatTreatment of patients with AK is recommended to achieve complete clearance and reduce the risk of progression to cSCC.14,55 However, given that spontaneous regression of AKs can occur in up to 23% of individual lesions,56 it is important to consider that the indication for treatment should be evaluated individually for each patient. Occasionally, it may be appropriate not to treat the patient immediately and opt for close monitoring of their evolution.
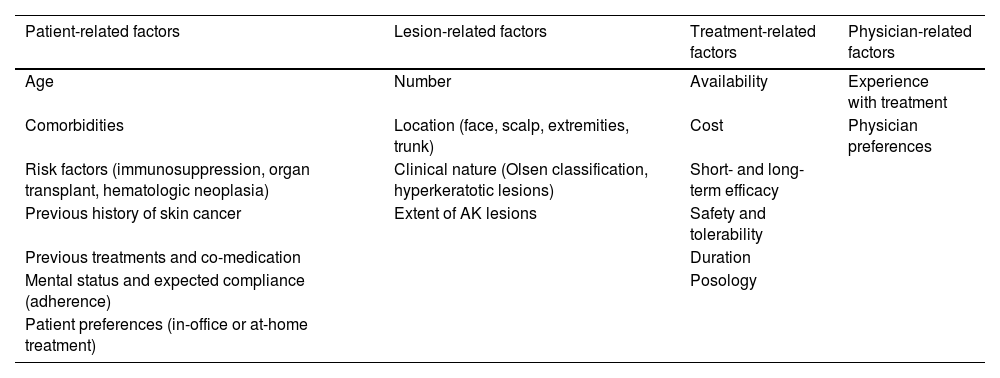
Aspects that should be considered when selecting treatmentThe choice of a treatment directed at the lesion or the field cancerization should take into account factors related to the patient, the lesions, treatment per se, and the physician (Table 2).2,12,57,58
Factors to consider in decision-making regarding AK treatment.
| Patient-related factors | Lesion-related factors | Treatment-related factors | Physician-related factors |
|---|---|---|---|
| Age | Number | Availability | Experience with treatment |
| Comorbidities | Location (face, scalp, extremities, trunk) | Cost | Physician preferences |
| Risk factors (immunosuppression, organ transplant, hematologic neoplasia) | Clinical nature (Olsen classification, hyperkeratotic lesions) | Short- and long-term efficacy | |
| Previous history of skin cancer | Extent of AK lesions | Safety and tolerability | |
| Previous treatments and co-medication | Duration | ||
| Mental status and expected compliance (adherence) | Posology | ||
| Patient preferences (in-office or at-home treatment) |
AK: actinic keratosis.
Cryosurgery should be offered for single or multiple AKs in limited numbers.2 Although its application is quick and simple, the application method is not standardized. It demonstrates complete cure rates of approximately 68%,59–61 also being effective outside the head and neck.62 Expected side effects are hypopigmentation and scars, and pain during application. In case of hyperkeratosis, it is recommended to remove it first to favor the effectiveness of the treatment.
Surgical excisionIndicated for single AKs if they are hyperkeratotic or hypertrophic, if they do not respond to other treatments, or if there is diagnostic uncertainty. The main advantage is the subsequent histological examination to exclude infiltrating cSCC. Disadvantages include the need for local anesthesia, bleeding, or unesthetic scarring.2
Destructive treatmentsChemical peels (trichloroacetic acid, Jessner's solution, phenol) have a lesion elimination rate of 31.9%.63 They are used for the treatment of field cancerization and have a photorejuvenating effect. With dermabrasion, when performed correctly, high and sustained complete response rates of 83–96% have been observed at the 2 year mark.64 However, it is not well standardized, is operator-dependent, and can cause unesthetic scars. Ablative lasers (CO2) have been used for specific lesions or in the context of rejuvenation associated with field treatment, but compared to cryotherapy, it is a more expensive, less available treatment with lower sustained clearance.6
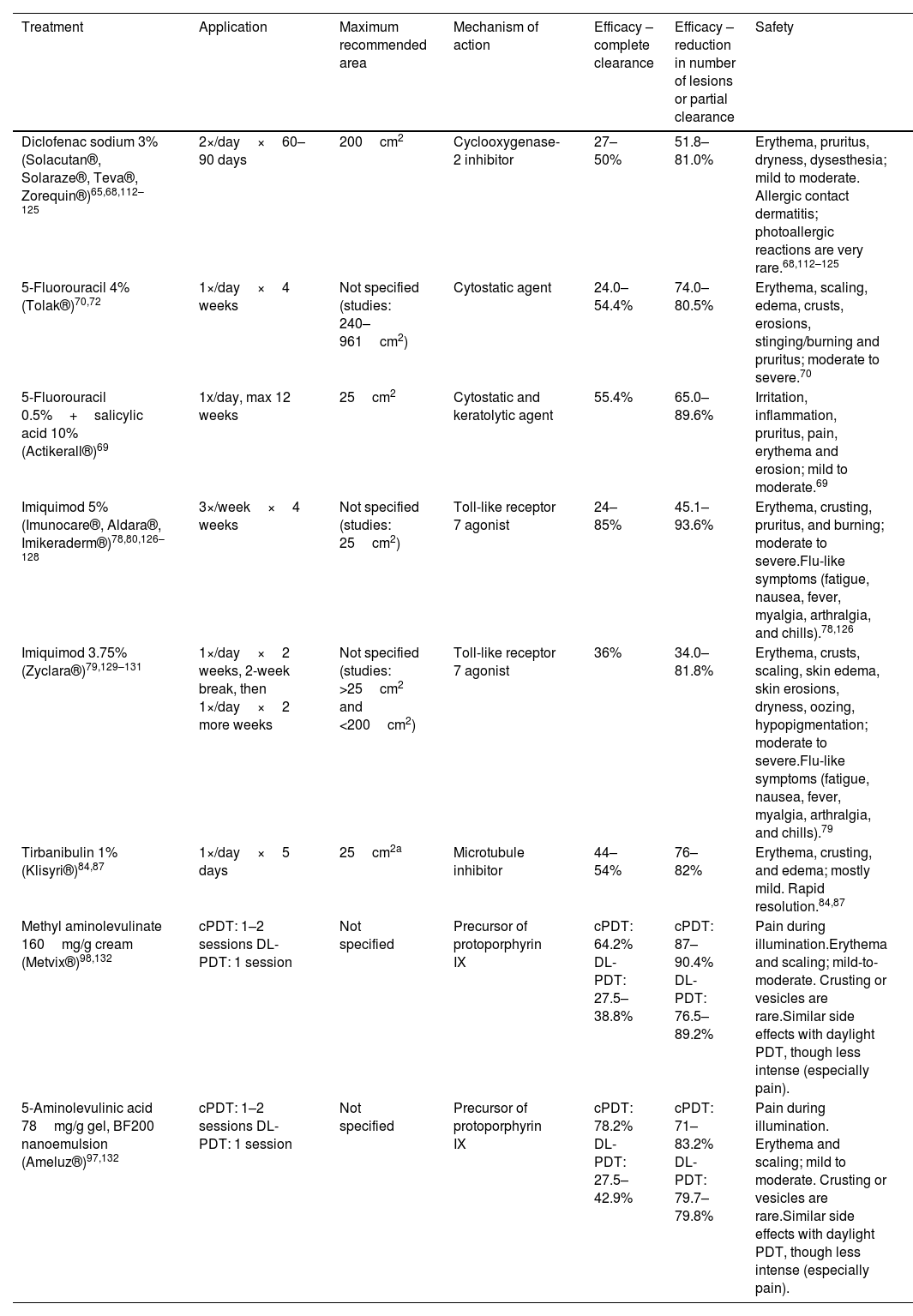
Topical treatmentsThe main characteristics of topical treatments are shown in Table 3.
Main characteristics of topical therapies currently available in Spain.
| Treatment | Application | Maximum recommended area | Mechanism of action | Efficacy – complete clearance | Efficacy – reduction in number of lesions or partial clearance | Safety |
|---|---|---|---|---|---|---|
| Diclofenac sodium 3% (Solacutan®, Solaraze®, Teva®, Zorequin®)65,68,112–125 | 2×/day×60–90 days | 200cm2 | Cyclooxygenase-2 inhibitor | 27–50% | 51.8–81.0% | Erythema, pruritus, dryness, dysesthesia; mild to moderate. Allergic contact dermatitis; photoallergic reactions are very rare.68,112–125 |
| 5-Fluorouracil 4% (Tolak®)70,72 | 1×/day×4 weeks | Not specified (studies: 240–961cm2) | Cytostatic agent | 24.0–54.4% | 74.0–80.5% | Erythema, scaling, edema, crusts, erosions, stinging/burning and pruritus; moderate to severe.70 |
| 5-Fluorouracil 0.5%+salicylic acid 10% (Actikerall®)69 | 1x/day, max 12 weeks | 25cm2 | Cytostatic and keratolytic agent | 55.4% | 65.0–89.6% | Irritation, inflammation, pruritus, pain, erythema and erosion; mild to moderate.69 |
| Imiquimod 5% (Imunocare®, Aldara®, Imikeraderm®)78,80,126–128 | 3×/week×4 weeks | Not specified (studies: 25cm2) | Toll-like receptor 7 agonist | 24–85% | 45.1–93.6% | Erythema, crusting, pruritus, and burning; moderate to severe.Flu-like symptoms (fatigue, nausea, fever, myalgia, arthralgia, and chills).78,126 |
| Imiquimod 3.75% (Zyclara®)79,129–131 | 1×/day×2 weeks, 2-week break, then 1×/day×2 more weeks | Not specified (studies: >25cm2 and <200cm2) | Toll-like receptor 7 agonist | 36% | 34.0–81.8% | Erythema, crusts, scaling, skin edema, skin erosions, dryness, oozing, hypopigmentation; moderate to severe.Flu-like symptoms (fatigue, nausea, fever, myalgia, arthralgia, and chills).79 |
| Tirbanibulin 1% (Klisyri®)84,87 | 1×/day×5 days | 25cm2a | Microtubule inhibitor | 44–54% | 76–82% | Erythema, crusting, and edema; mostly mild. Rapid resolution.84,87 |
| Methyl aminolevulinate 160mg/g cream (Metvix®)98,132 | cPDT: 1–2 sessions DL-PDT: 1 session | Not specified | Precursor of protoporphyrin IX | cPDT: 64.2% DL-PDT: 27.5–38.8% | cPDT: 87–90.4% DL-PDT: 76.5–89.2% | Pain during illumination.Erythema and scaling; mild-to-moderate. Crusting or vesicles are rare.Similar side effects with daylight PDT, though less intense (especially pain). |
| 5-Aminolevulinic acid 78mg/g gel, BF200 nanoemulsion (Ameluz®)97,132 | cPDT: 1–2 sessions DL-PDT: 1 session | Not specified | Precursor of protoporphyrin IX | cPDT: 78.2% DL-PDT: 27.5–42.9% | cPDT: 71–83.2% DL-PDT: 79.7–79.8% | Pain during illumination. Erythema and scaling; mild to moderate. Crusting or vesicles are rare.Similar side effects with daylight PDT, though less intense (especially pain). |
cPDT: conventional photodynamic therapy; DL-PDT: daylight photodynamic therapy.
Use over a maximum area of 100cm2 is currently under study. Phase 1 MUST study results have been published with favorable outcomes.91
Diclofenac has anti-inflammatory and antineoplastic action. The 3% gel presentation of hyaluronic acid has been approved since 201165 for treating single or multiple AKs and field cancerization (including large fields). Due to its long posology (60–90 days), adherence to treatment may not be as expected.66 Complete response rates are around 41%.67 Tolerance is generally good, although it is associated with pruritus in one-third of patients, and there is a risk of photosensitivity and contact dermatitis.68
5-FluorouracilThe formulations marketed in Spain are 5-fluorouracil (5-FU) 0.5% with 10% salicylic acid in cutaneous solution (since 2013)69 and 5-FU 4% cream (since 2020).70 Both have been approved for treating single or multiple AKs and field cancerization.
The classic formulation of 5-FU 5% cream was administered twice daily for 21 days. A randomized clinical trial with 624 patients comparing 4 types of treatments for AKs (5-FU 5%, imiquimod 5%, ingenol mebutate [IMB], and photodynamic therapy [PDT]) concluded that 5-FU 5% was the most effective treatment with a probability of remaining disease-free of 74.7% 1 year into therapy.71 However, it is associated with high rates of local skin reactions (40% erosions and 57% crusts71), which forces treatment discontinuation in up to one-third of patients, impacting efficacy. Off-label, it is used in shorter administration regimens (10–15 days once daily) with reasonable response rates and better tolerance, although these regimens have not been evaluated in clinical trials nor are there studies with real-world data. The marketed 5-FU 4% cream shows similar efficacy and better tolerability than 5% when applied once daily for 4 weeks.72
5-FU 0.5% with 10% salicylic acid has a keratolytic effect that enhances the cytostatic effect of 5-FU. Although it is applied once daily, treatment can last up to 12 weeks, which may compromise adherence. It is especially indicated for hyperkeratotic AKs.
The application of 5-FU 5% along with calcipotriol 0.005% twice daily for 4 days is more effective than 5-FU monotherapy according to a randomized clinical trial, although complete clearance was only achieved in 27% of participants on week 8.73 The combination of calcipotriol and 5-FU reduced the risk of cSCC on the face and scalp over a 3-year period.73–76
The use of occlusive treatment with 5-FU (application of 5-FU and zinc oxide and elastic bandage, changed once a week) has been used for the treatment of large fields on the upper and lower limbs with good results.77
ImiquimodImiquimod has antiviral and antineoplastic activity in addition to stimulating the innate and acquired immune response. It is a TLR7 receptor agonist and produces a local elevation of IFN-alpha levels among other cytokines. In Spain, 5% imiquimod (since 1999)78 and 3.75% imiquimod (since 2012)79 are marketed. The 2 formulations have been approved for treating single or multiple AKs and field cancerization. The main differences between the 2 are the extent of the field to be treated and posology (Table 3). Imiquimod 5% cream achieves complete response rates in 45–57% of patients.80,81 At 1 year, 53.9% of patients remain disease-free.71 A total of 15% discontinue treatment due to local skin reactions, almost half develop erosions or ulcers, and 70% develop crusts.71 Imiquimod has been associated with systemic symptoms in the form of a flu-like syndrome and the development of a local skin reaction beyond the treated areas. Furthermore, 5% imiquimod cream has also been used in 12 consecutive day treatments with complete response rates of 52.3% and partial response rates of 75.4%.82
Ingenol mebutateIn 2020, the European Medicines Agency (EMA) suspended the approval of IMB for AK due to the observation of a higher incidence rate of cSCC in patients on IMB vs those on imiquimod in a 3-year safety study of 484 patients (3.3% vs 0.4%).83 Patients previously on IMB should be monitored for the appearance of skin tumors in previous treatment fields.
TirbanibulinSince 2021, tirbanibulin has been approved for treating single or multiple non-hypertrophic AKs and field cancerization on the face or scalp in an area of up to 25cm2.84 It uses a novel mechanism of action that inhibits tubulin polymerization during cell division, causing the cell to undergo apoptosis and not necrosis, which has been associated with high efficacy with mostly mild to moderate local reactions.85–88 It is administered once daily for 5 consecutive days, with mild local skin reactions, very high adherence, and satisfaction of physicians and patients.89 It achieves complete remission rates of 49% and partial response rates>70%.87,90 The safety and efficacy profile of tirbanibulin in larger areas (up to 100cm2) are being evaluated, with favorable results obtained in a phase 1 study, results that have been recently confirmed in a phase 3 study.91,92 Erythema is the only frequent local skin reaction, with a maximum peak at 8 days and complete resolution at 30 days without the need for intervention. Pain, itching, and burning sensation are observed in <10%, as are erosions, which are rated as moderate or severe in 3% of patients.87
Photodynamic therapyPDT is based on the application of a topical photosensitizing agent together with a light source to selectively destroy atypical keratinocytes. Multiple PDT regimens have been established, making it a heterogeneous procedure.93–96 All types of PDT are offered to treat single or multiple AKs and field cancerization.
Photosensitizers and indications approved in Spain are methyl aminolevulinate cream (MAL) since 200298 for non-hypertrophic AKs; and 5-aminolevulinic acid in nanoemulsion gel (ALA-BF200) since 201297 for mild and moderate AKs and field cancerization. Both can be used in PDT with red light as well as with daylight.
PDT may require pretreatment of the AK94 with 10% salicylic petrolatum for 1 week prior to therapy, 20% urea, chemical peeling, ablative laser, or microneedling.
Conventional protocol consists of the application of the photosensitizer, with incubation under occlusion for 2–3h before activation with red light (37.5J/cm2) for 10–20min. Complete clearance rates, between 31.4% and 91.0%; lesion reduction rate, between 58.0% and 94.3%.2,6,55 A total of 37.7% of responding patients remain disease-free 1 year into therapy.71 PDT is associated with pain in 80% of patients and moderate/intense burning in 86%. The application of local cold, intake of non-steroidal anti-inflammatory drugs 30min before irradiation, fractionated irradiation with 3-min pauses, the application of topical clobetasol, “talkesthesia,” or the infiltration of local or truncal anesthesia without vasoconstrictor help to improve tolerance.94
Daylight PDT protocols use natural sunlight as the energy source through the application of the photosensitizer and exposure to sunlight in the following 30min for 2h.99 Complete clearance rates vary between 27.5 and 42.9% when using ALA (lesion reduction rate, 79.7–79.8%) and 27.5–38.8% for MAL (lesion reduction rate, 76.5–89.2%).2,6,55 Advantages include less pain, the possibility of treating large fields, greater compliance, and the lack of need for artificial light sources, which reduces costs.100 This modality can be performed in Spain throughout the year due to our latitude, except on rainy days or if the temperature is <10°C.
Combined PDT, performed by carrying out daylight PDT for 2h followed by the application of red light at the usual dose, has shown better efficacy vs daylight alone (complete response, 31.6% vs 15.8% and partial response, 63.2% vs 25.3%) with a mild/moderate increase in local side effects and pain.101
Combination therapyThe combination of one modality to treat the lesion with another to treat field cancerization in the same patient is common in the routine clinical practice with the aim of maximizing response, improving efficacy and tolerance.6,55 Combinations used include the combination of ablative laser as a pretreatment to PDT,102 cryosurgery plus topical therapy,103 and PDT plus topical therapy, including calcipotriol 15 days before, 5-FU 5% twice daily 6–7 days before, imiquimod before or after PDT, or tazarotene before PDT.104,105
Other treatmentsOther treatments have been described for the management of AKs in patients with special severity, such as volumetric modulated arc therapy (VMAT) and oral capecitabine, although their use is not widespread in clinical practice in Spain.
Some studies have evaluated the use of VMAT to treat field cancerization in patients with multiple AKs and even squamous cell carcinoma. The decision to use this more aggressive therapy, for example, to treat multiple lesions on the scalp, should be evaluated by a multidisciplinary team of dermatology and radiation oncology.106,107
Capecitabine can be used in the context of cSCC prevention. However, due to its side effects, it is a rarely used treatment in our setting and is reserved for high-risk cases, such as solid organ transplant recipients with a history of multiple squamous cell carcinomas.108
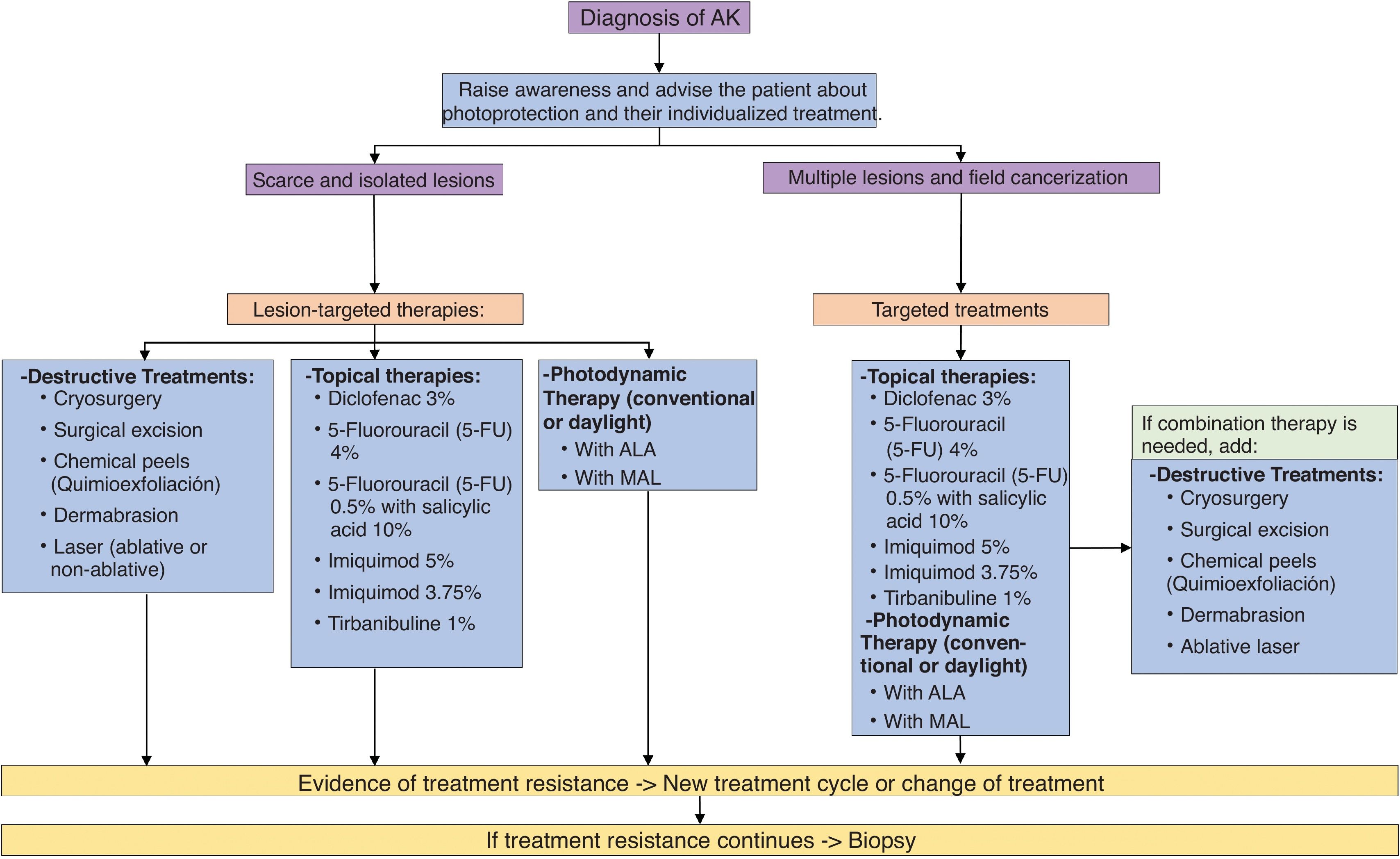
Summary of AK management at presentIn recent years, new drugs and new formulations have been approved for the treatment of AK. Fig. 3 illustrates the updated treatment algorithm for AK proposed in 2014 by Ferrandiz et al.14 according to the treatments currently available in Spain.
Special patients: immunosuppression and organ transplantationChemoprophylaxis with oral retinoids is effective for the prevention of AK and cSCC in this at-risk population.51
A systematic review of local treatments for AK in 242 KTRs found that PDT had the highest lesion clearance rates (46–100%), followed by 5-FU (79%), imiquimod (61–73.7%), and diclofenac (53%); but there are no comparative studies and this review included only 1 study with 5-FU.109 In general, the efficacy of treatments in KTRs seems to be lower than in immunocompetent patients, and experts in the treatment of transplant patients have not reached a consensus on which AK treatment would be the first choice in this population.110
Due to the more aggressive course of the disease and a higher proportion of treatment-resistant lesions, it is often necessary to repeat treatment.55
Follow-upRegular follow-up visits should be scheduled as the patient with AK should be considered a chronic patient. Follow-up after initial treatment involves evaluating the success of the treatment, the recurrence of lesions, and the development of new AKs. Self-examination and photoprotection measures should be emphasized. The follow-up interval should be adapted to the profile of each patient and the specific risk of developing carcinoma.6,14,55 No studies have demonstrated a more appropriate follow-up protocol. It has been shown that the preventive effect of field cancerization treatments appears within the first year but is lost in the long term. Thus, since AK is considered a chronic disease, it is recommended to perform periodic treatments to eliminate newly appearing lesions and reduce the risk of cSCC.111
- 1.
The diagnosis of AK is fundamentally clinical, although techniques such as dermoscopy, RCM, or OCT can help us confirm the diagnosis. Skin biopsy is reserved for doubtful cases.
- 2.
Currently, we have several systems of clinical, microscopic, and affected area classification, without a clear correlation between them, but fundamental for a more adequate assessment of the patient's risk.
- 3.
It is not possible to predict which AK will progress to cSCC or when, so treatment of all AKs is recommended.
- 4.
There is a wide range of treatments available for AKs, to which new approvals have been added in recent years, such as daylight PDT administration, 4% 5-fluorouracil formulation, and 1% tirbanibulin, among others.