Despite the large number of articles published on skin lesions related to COVID-19, clinicopathological correlation has not been performed consistently and immunohistochemistry to demonstrate spike 3 protein expression has not been validated through RT-PCR.
Material and methodsWe compiled 69 cases of patients with confirmed COVID-19, where skin lesions were clinically and histopathologically studied. Immunohistochemistry (IHC) and RT-PCR was performed in skin biopsies.
ResultsAfter a careful review of the cases, 15 were found to be dermatosis not related to COVID-19, while the rest of the lesions could be classified according to their clinical characteristics as vesicular (4), maculopapular eruptions (41), urticariform (9), livedo and necrosis (10) and pernio-like (5). Although histopathological features were similar to previously reported results, we found two previously unreported findings, maculopapular eruptions with squamous eccrine syringometaplasia and neutrophilic epitheliotropism.
IHC showed in some cases endothelial and epidermal staining but RT-PCR was negative in all the tested cases. Thus, direct viral involvement could not be demonstrated.
ConclusionsDespite presenting the largest series of confirmed COVID-19 patients with histopathologically studied skin manifestations, direct viral involvement was difficult to establish. Vasculopathic and urticariform lesions seem to be those more clearly related to the viral infection, despite IHC or RT-PCR negative results failed to demonstrate viral presence. These findings, as in other dermatological areas, highlight the need of a clinico-pathological correlation to increase knowledge about viral involvement in COVID-19 skin-related lesions.
A pesar del gran número de artículos publicados sobre las lesiones cutáneas relacionadas con la COVID-19, no se ha realizado una correlación clinicopatológica de manera consistente, y no ha sido validado el estudio de inmunohistoquímica para demostrar la expresión de la proteína spike 3 mediante RT-PCR.
Material y métodosRecopilamos 69 casos de pacientes con COVID-19 confirmada, en los que se estudiaron las lesiones cutáneas a nivel clínico e histopatológico, habiéndose realizado la prueba inmunohistoquímica (IHQ) y RT-PCR en las biopsias cutáneas.
ResultadosTras una revisión detallada de los casos, en 15 de ellos se encontró que la dermatosis no guardaba relación con la COVID-19, mientras que el resto de las lesiones podrían clasificarse de acuerdo con sus características clínicas como vesiculares (4), erupciones maculopapulares (41), urticariformes (9), livedo y necrosis (10) y de tipo perniosis (5). Aunque las características histopatológicas fueron similares a los resultados previamente reportados, encontramos dos hallazgos no reportados previamente: erupciones maculopapulares con siringometaplasia ecrina escamosa y epiteliotropismo neutrofílico.
La IHQ reflejó en ciertos casos tinción endotelial y epidérmica, pero la prueba RT-PCR fue negativa en todos los casos probados. Por ello no pudo demostrarse el compromiso viral directo.
ConclusionesA pesar de presentar la mayor serie de pacientes con COVID-19 confirmada y manifestaciones cutáneas histopatológicamente estudiadas, el compromiso viral directo fue difícil de establecer. Las lesiones vasculopáticas e urticariformes parecen ser las más claramente relacionadas con la infección viral, a pesar de que los resultados negativos de la IHQ o RT-PCR no pudieron demostrar la presencia viral. Dichos hallazgos, como en otras áreas dermatológicas, subrayan la necesidad de una correlación clinicopatológica para incrementar el conocimiento sobre la implicación viral en las lesiones cutáneas relacionadas con la COVID-19.
The coronavirus SARS-CoV-2 causes a broad-spectrum disease, coronavirus disease 2019 (COVID-19), primarily involving the respiratory tract, although many extrapulmonary manifestations may occur, caused by either direct dissemination or by immunopathological manifestations. The first article highlighting cutaneous involvement of COVID-19 was published early in the pandemic and was followed by many other series, in which skin involvement ranged from 0.2 to 20% of cases.1–4
Despite the large number of published articles related to cutaneous involvement in COVID-19, clinicopathological correlation has not been performed consistently.2,5–9 As an example, Freeman et al., in a study including 716 patients from 31 countries, showed only 14 cases with biopsies.6
It is also noteworthy the lack of a comprehensive study of the presence of the virus in skin biopsies either with immunohistochemistry (IHC) or other techniques such as in situ hybridization (ISH) or RNAscope.10
Since Spain was severely affected by COVID-19, which claimed the lives of more than 5.3 million people in the world, we established a national working group to compile the experience of different hospitals. With this information we published at the end of April of 2020 an article proposing 5 different types of cutaneous lesions related to COVID-19, vesicular eruptions (9%), maculopapular eruptions (47%), urticariform lesions (19%), livedo and necrosis (6%) and pernio-like lesions (19%).11
We report here a further histopathological study aimed at gaining insight into the pathogenesis of the different COVID-19 related dermatoses and at establishing whether direct viral involvement can be demonstrated in some of them by validating IHC with RT-PCR.
Material and methodsWe compiled 69 cases of skin lesions with a complete clinical history and skin biopsy from 6 different Spanish hospitals. We reviewed the clinical chart and every biopsy to find a proper diagnosis. All biopsies were fixed in 10% buffered formaldehyde and paraffin-embedded according to guidelines. Four-micron thick slices of paraffin-embedded tissue were used for routine hematoxylin/eosin staining. Immunohistochemistry with anti-SARS-CoV-2 spike protein antibodies was performed in Hospital Universitario de la Princesa and in Universitäts-Hautklinik Münster. RT-PCR and RNAscope were performed as recommended to corroborate in situ detection.12
InmunohistochemistryIn all our skin biopsies immunohistochemistry was performed with anti-SARS-CoV-2 spike protein antibodies (clones 1A9 and GTX135335, GeneTex, California, USA).
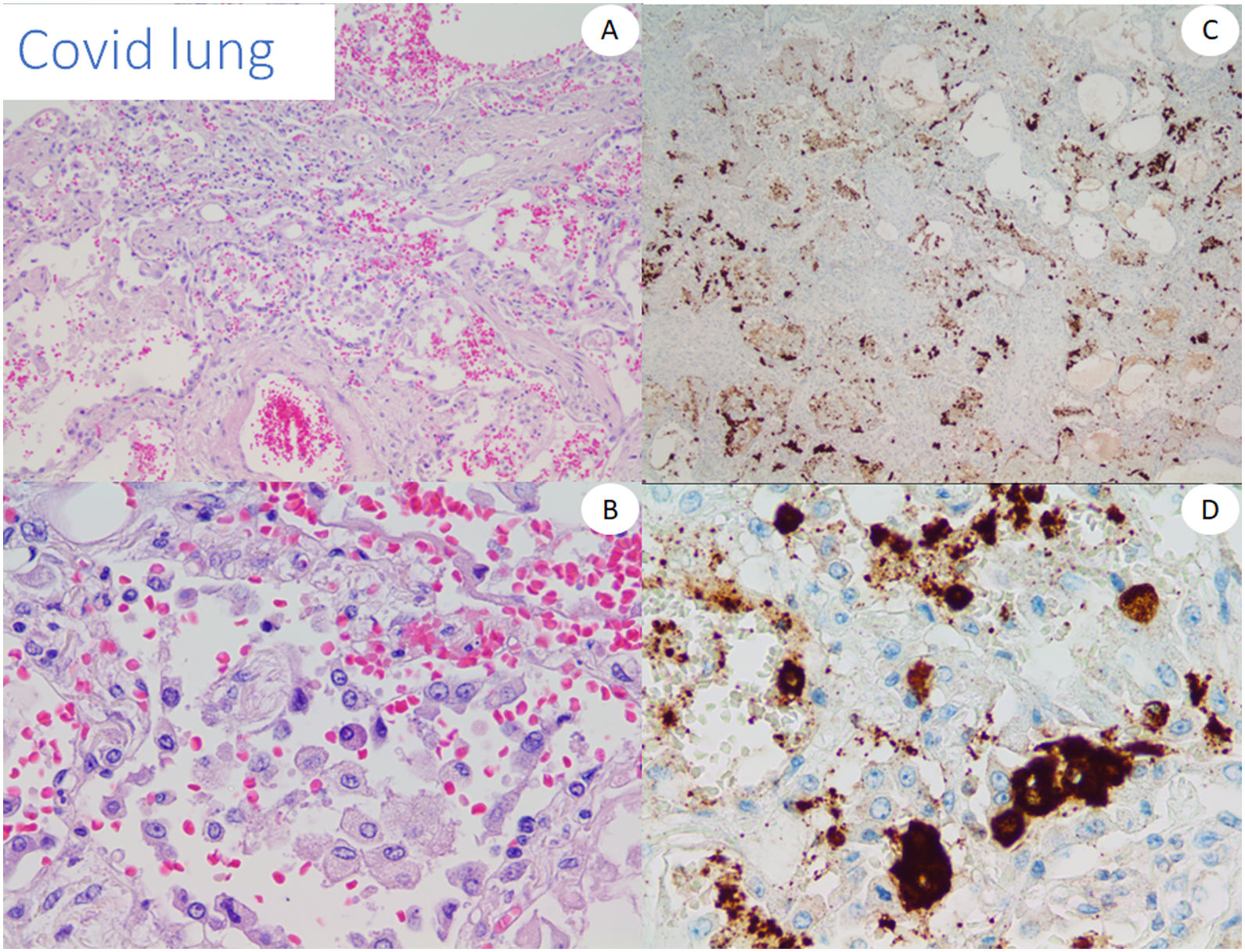
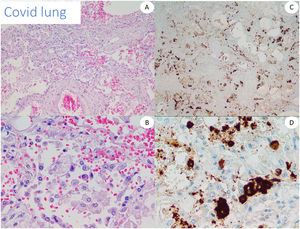
We used lung tissue from deceased COVID-19 patients as positive control of our anti-spike 3 antibody (Fig. 1) and as control cases. In addition, we tested the antibody by staining 5 cases of conventional pernio taken from our archives from a pre-pandemic period.
RT-PCR was performed for all the cutaneous biopsies, ISH was also performed with RNAscope® Probe-V-nCoV2019-S, Cat. No. 848561, Manual Assay 2.5.
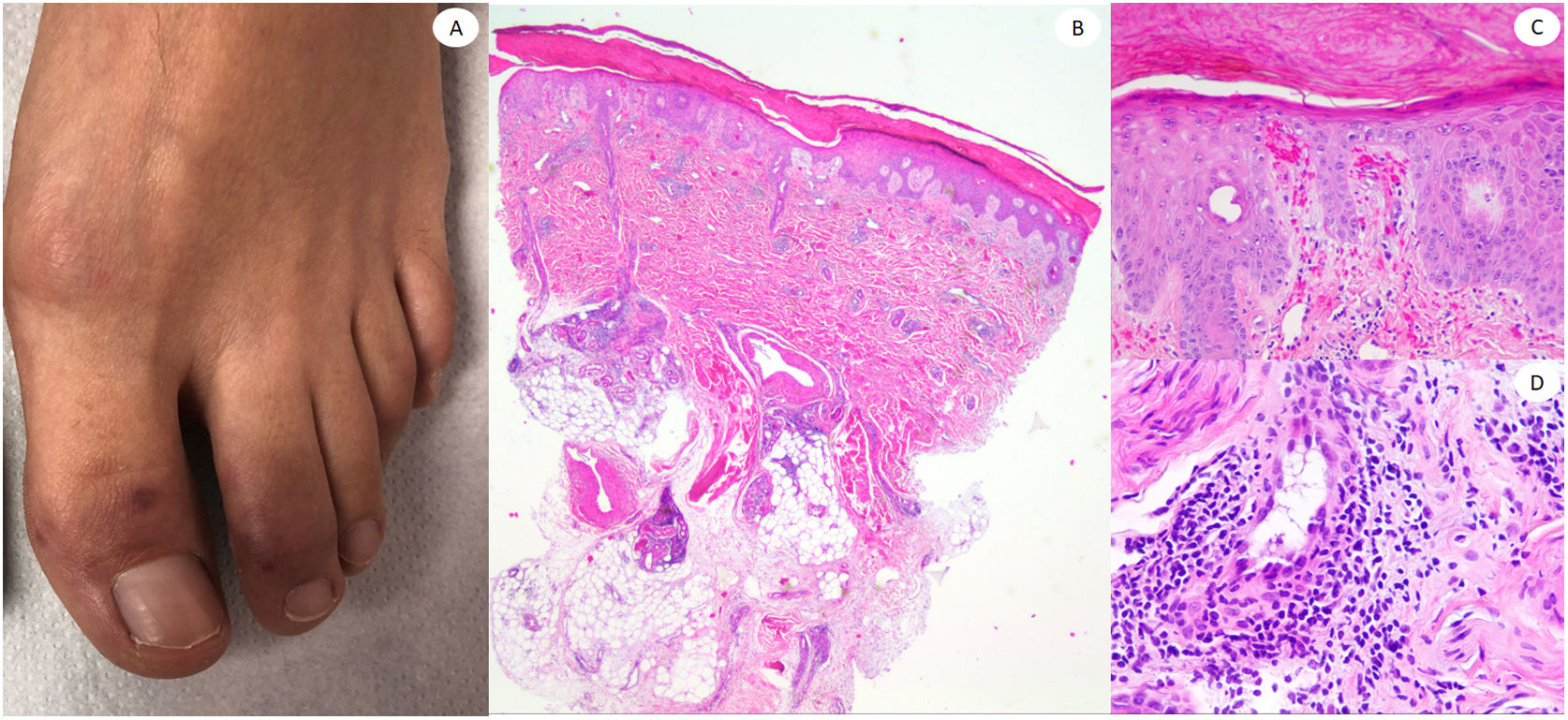
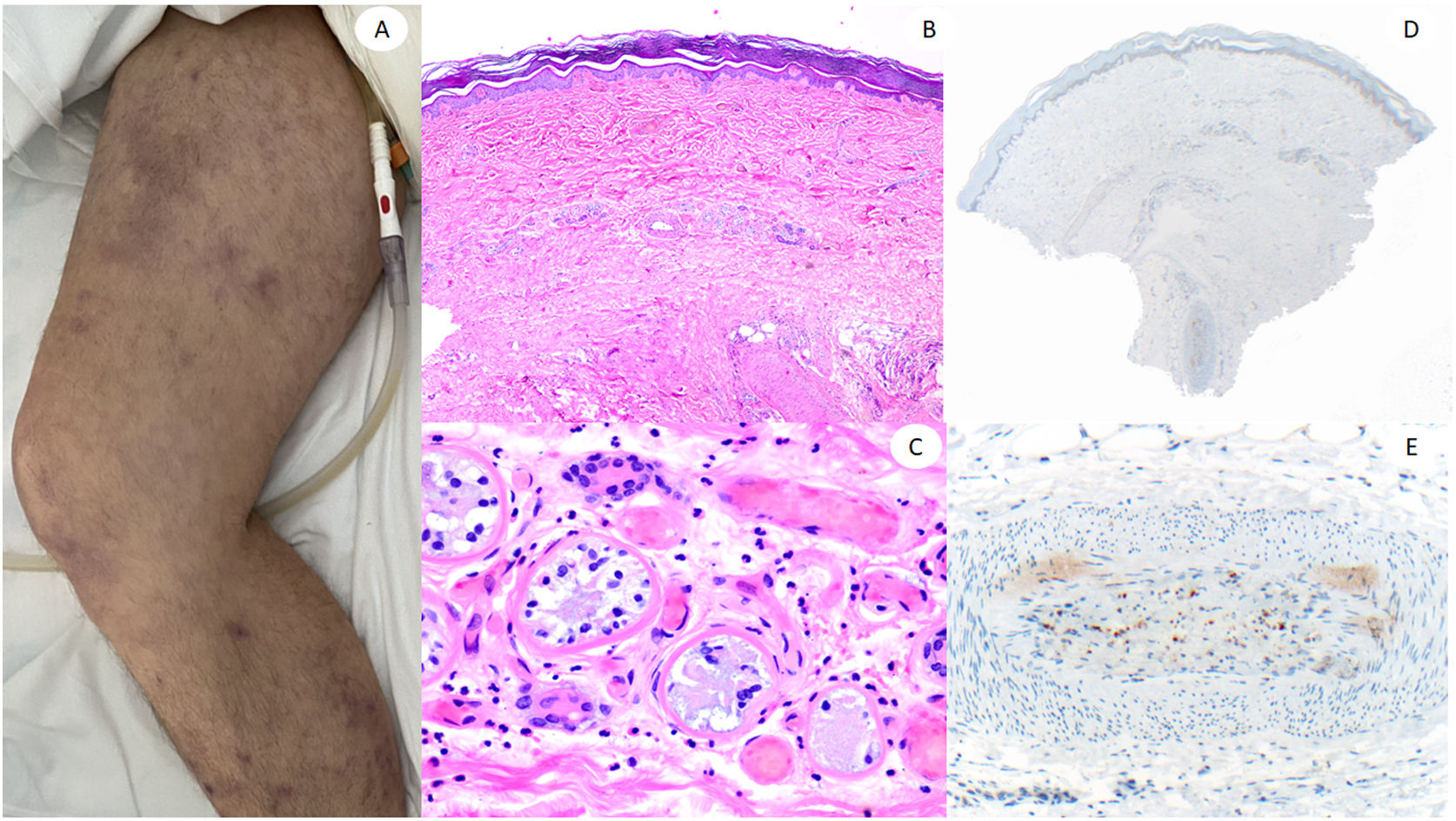
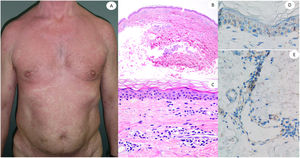
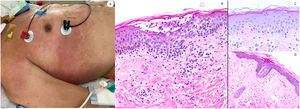
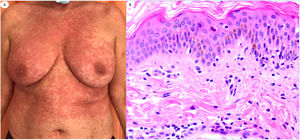
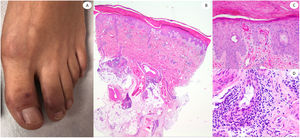
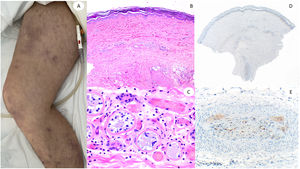
ResultsLesions of the cases were classified according to their clinical characteristics as vesicular (4), maculopapular eruptions (41) (Figs. 2 and 3), urticariform (9) (Fig. 4) livedo and necrosis (10) (Fig. 5) and pernio-like (5) (Fig. 6).
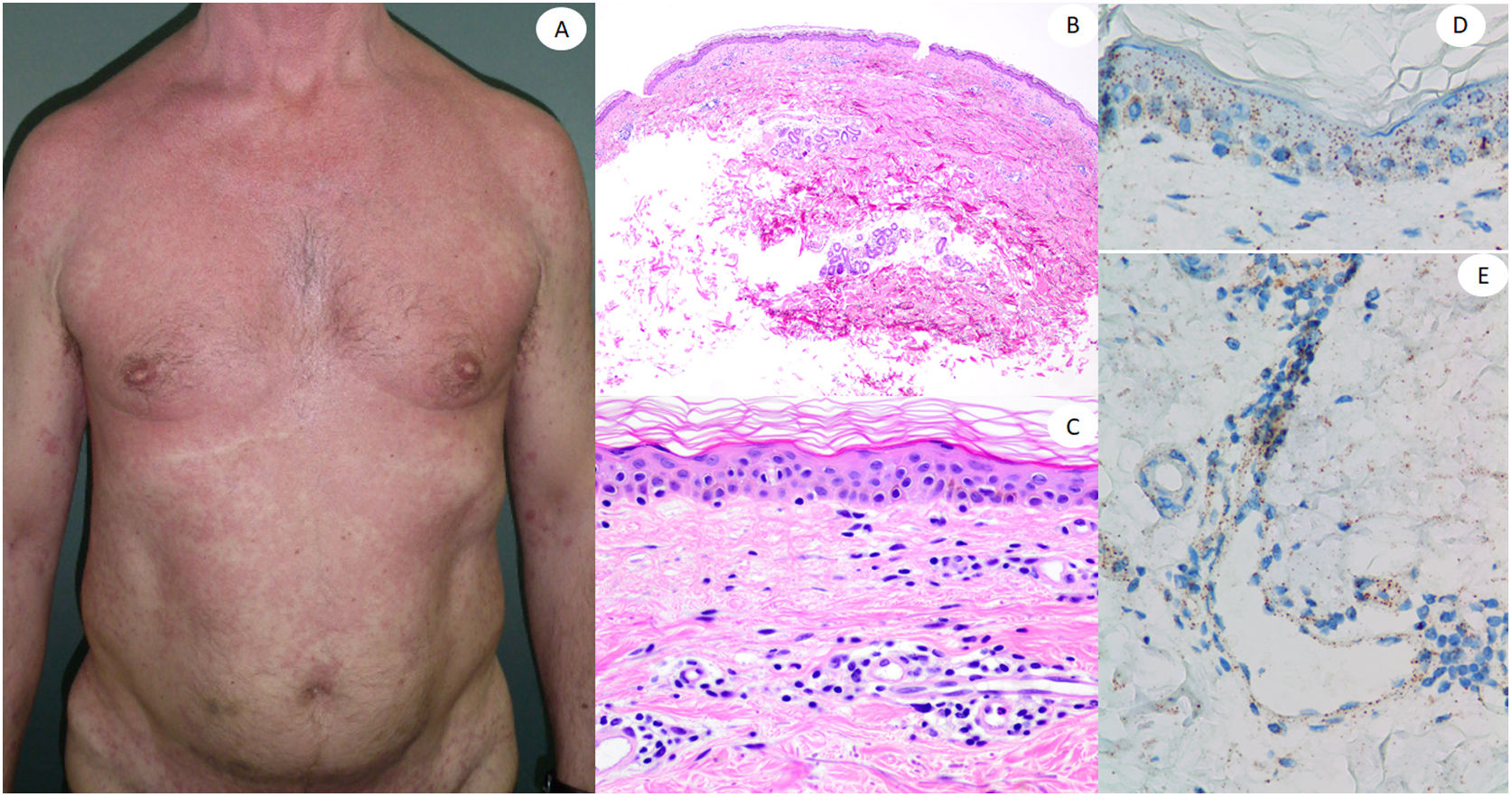
Maculopapular reaction in a 53-year-old male involving the trunk and extremities. Histopathological pictures show a normal flattened epidermis, as well as a scarce lymphocytic perivascular infiltrate. Anti-spike 3 IHC shows a granular staining mostly located in the epidermis and in the endothelia, but also intermingled within the inflammatory infiltrate.
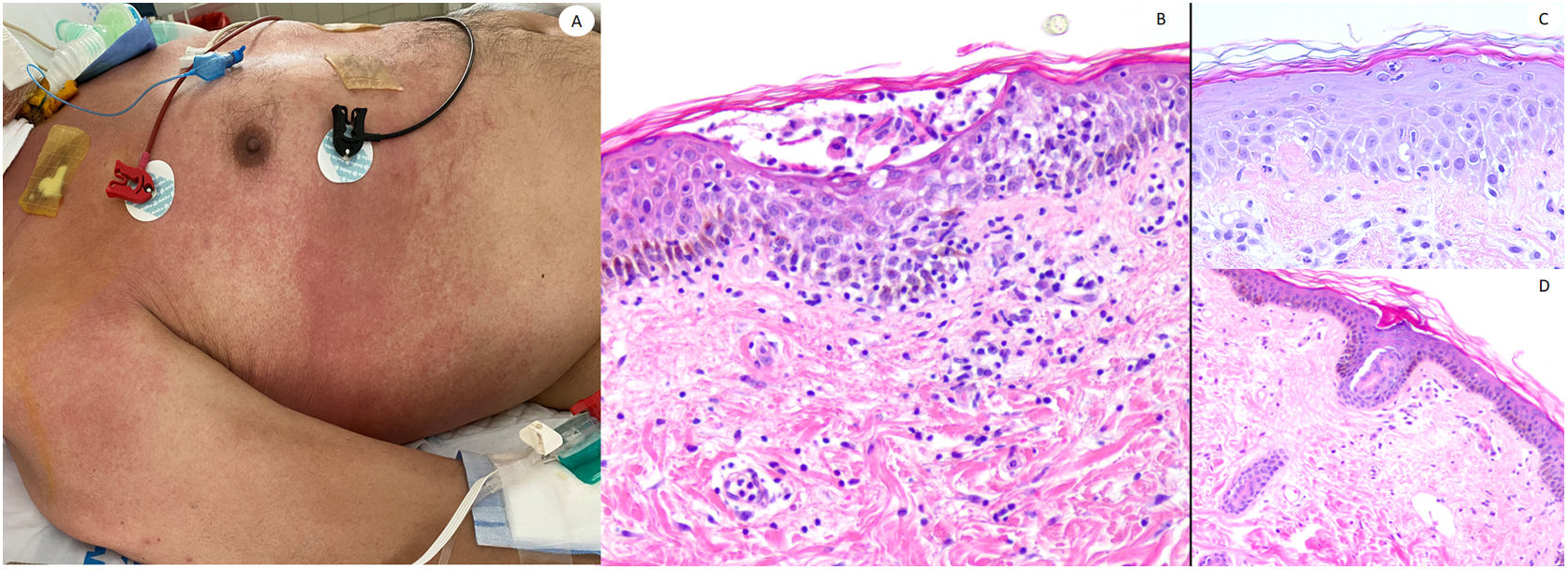
Maculo-papular reaction in a patient in intensive care unit showing confluent erythematous and purplish papules mostly involving flanks and bank and with scarce vesicles. Histopathology shows an intraepidermal collection of neutrophils and Langerhans cells. A slight vacuolar interphase as well as a light perivascular lymphocytic infiltrate are shown. Right panel shows neutrophilic epitheliotropism (upper picture) and eccrine syringometaplasia in clinically similar cases, where few neutrophils can be observed in the upper epidermis without conforming a pustule.
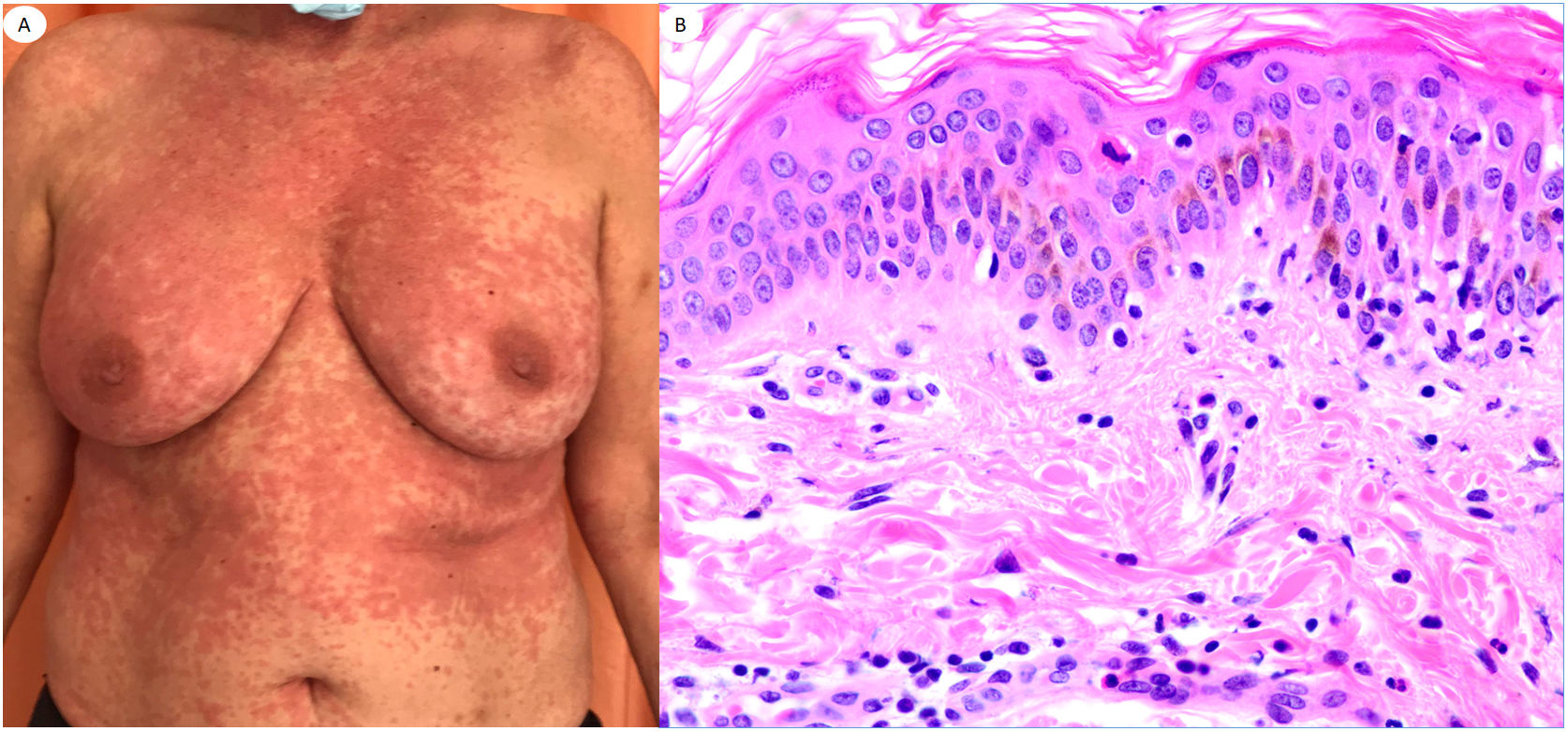
Urticariform reaction in a 62-year-old female showing scarce perivascular inflammatory infiltrate with polymorphonuclear (PMN) cells and few eosinophils, vacuolar degeneration, epitheliotropic PMN and few apoptotic keratinocytes. Lesions appeared at home, after the patient with COVID-19 pneumoniae was discharged from the hospital.
60-Year-old male, in intensive care unit due to bilateral COVID-19 pneumonia, with reticular lesions in both legs. Histopathology shows eccrine gland necrosis as well as the presence of red thrombi within the reticular dermis vessels. Right side shows anti-spike 3 where granular staining can be observed in an area in which a dermo-hypodermal vessel is occluded by a neutrophil-rich thrombi.
COVID-19 was confirmed in all the cases with PCR or antigen test in swab samples from nasal or pharyngeal area. All the biopsies were evaluated for histopathological pattern, parakeratosis, spongiosis, dyskeratosis, epidermal necrosis, subcorneal pustules, vacuolar damage, lichenoid infiltrate and presence of edema, thrombi or other vasculitic involvement.
The following main histopathological patterns were observed: neutrophils within papillary or subcorneal dermis (in 14 cases); interstitial (3), eczematous (7) or urticariform pattern (6), vasculitis (8), neutrophilic dermatosis (3), pernio-like (5), and lymphocytic superficial dermatitis (8).
After careful clinicopathological correlation we reclassified 15 of the biopsies as exudative erythema multiforme (EEM) (1), Grover disease (1), herpes simplex and zoster infections (3), mycosis (1), perifolliculitis (1), atypical mycobacterial disease (1) and disseminated zoster (1). In six of the cases acute generalized exanthematic pustulosis (AGEP) fitted even better than maculopapular eruptions and a drug was found as a plausible trigger. In all the other skin biopsies, clinicopathological correlation led us to a final diagnosis of COVID-19-associated skin lesions.
No immunostaining differences were found between conventional and COVID-19 pernio cases, only granular staining in different quantity, which sometimes was absent in both groups, although it tended to be more striking in epidermis and within inflammatory infiltrates, mostly if endothelia were inflamed. We did not find a clear cytoplasmic staining as all the staining seemed to be artifacts.
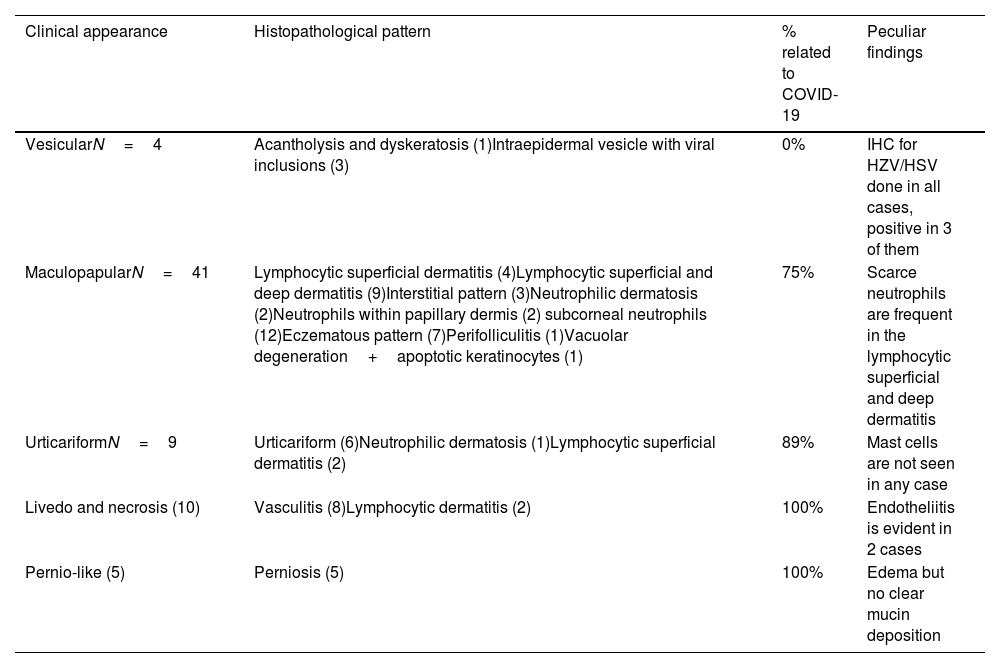
Table 1 shows the main clinical and histopathological findings in the series.
Clinical and histopathological pattern, as well as percentage of cases directly related to COVID-19 infection.
| Clinical appearance | Histopathological pattern | % related to COVID-19 | Peculiar findings |
|---|---|---|---|
| VesicularN=4 | Acantholysis and dyskeratosis (1)Intraepidermal vesicle with viral inclusions (3) | 0% | IHC for HZV/HSV done in all cases, positive in 3 of them |
| MaculopapularN=41 | Lymphocytic superficial dermatitis (4)Lymphocytic superficial and deep dermatitis (9)Interstitial pattern (3)Neutrophilic dermatosis (2)Neutrophils within papillary dermis (2) subcorneal neutrophils (12)Eczematous pattern (7)Perifolliculitis (1)Vacuolar degeneration+apoptotic keratinocytes (1) | 75% | Scarce neutrophils are frequent in the lymphocytic superficial and deep dermatitis |
| UrticariformN=9 | Urticariform (6)Neutrophilic dermatosis (1)Lymphocytic superficial dermatitis (2) | 89% | Mast cells are not seen in any case |
| Livedo and necrosis (10) | Vasculitis (8)Lymphocytic dermatitis (2) | 100% | Endotheliitis is evident in 2 cases |
| Pernio-like (5) | Perniosis (5) | 100% | Edema but no clear mucin deposition |
Abbreviations: HSV: herpes simplex virus; HZV: herpes zoster virus; IHC: immunohistochemistry.
All the biopsies tested with RT-PCR and RNAscope were negative for COVID-19. Immunohistochemical results for the viral spike protein differed with the different antibodies. With the 1A9 clone we were not able to find any vascular staining, and only granular staining was seen within the inflammatory infiltrates. On the other hand, with the GTX135335 clone some cases showed a diffuse staining of small particles that was interpreted as background, while the main finding was the presence of a more intense granular deposition in no particular structure in the epidermis and endothelia, the latter mostly in the cases with neutrophilic infiltrates and in those with vasculopathy. As the pattern was not conclusive, we found difficult to make a clear interpretation of positivity in these cases.
DiscussionThe largest series studying skin biopsies in patients with COVID-19 was recently published by Quintero-Bustos et al., compiling 41 cases. They found IHC endothelial staining for the spike protein in every type of skin lesion, although they mostly included pityriasis lichenoides chronica (PLC) and pityriasis lichenoides et varioliformes acuta (PLEVA) as well as vesicular lesions. However, we want to highlight that the main limitation of this study was the absence of both clinical pictures and RT-PCR to confirm the viral presence.10
Our results on maculo-papular skin eruptions are aligned with those previously reported by our group.13 In our previous study as they were mainly characterized by a mild to dense superficial perivascular lymphocytic infiltrate sometimes with focal vacuolar degeneration,13 and rarely squamous syringometaplasia either epitheliotropic was observed in one case each in this series, both no previously reported findings. Regarding the cases mimicking AGEP, hydroxychloroquine and other drugs are usually involved in these types of cases.14 We find difficult to exclude a drug eruption, since the Spanish group (COVID-PIEL) reported drug intake in up to 84% of affected patients15 and these lesions predominate in the posterior or recumbent regions.5
Our negative IHC and ISH results for the spike protein are aligned with the previously reported negative findings of Fattori et al.16 Only a subtle granular pattern was seen in the epidermis and along the vessels.
Urticarial lesions, are considered early, non-specific reactions, where drugs or other viral reactivations may be involved.17 Histopathologically, they are reported as conventional urticaria, with different degrees of edema as well as scarce lymphocytes and eosinophils, while one case presented epitheliotropism of neutrophils, a finding not previously reported. Some authors reported consistent vacuolar degeneration in their cases as a clue for COVID-19.5 In our series, although urticarial lesions seemed to be temporally related to the viral infection, our IHC and RT-PCR studies were not able to demonstrate a direct viral involvement.
All the cases included as vesicular lesions related to COVID-19 were reclassified as other diagnosis in our series, mostly as different herpes-related lesions (3/4). We agree with Mahé et al., who considered those lesions as histologically different from varicella.18 In our series and in previously published articles, some cases fitted with the so-called vesicular Grover or pseudoherpetic Grover.19
Our cases of pernio-like lesions fitted with the so called covid toes, which mostly appear in the feet of young patients, although they can be present also in adults. In our series this lesion was infrequent, as in other series where COVID-19 inpatients predominate.5 All our cases exhibited dense superficial and deep lymphocytic infiltrates, some of them with vascular involvement as in the series of 24 cases by Kolivras et al.20 However, none of our cases exhibited striking mucin deposition as described by Giavedoni et al.21 We did not find any case resembling EEM as Torrelo et al. reported,22 and we were not able to find anti-spike 3 positivity in the eccrine glands as another Spanish group did.23 Our results are aligned with the negative IHC findings with anti SARS-CoV-2 nucleocapsid antibody in 6 cases published by Ko et al., although we used a different antibody in our series.24
Finally, as other authors, we found the more consistent results in the livedoid and necrotic lesions, mostly occurring in intensive care patients, where admixed findings of thrombosis and neutrophilic vasculitis were found. We have not followed the patients to establish mortality, which has been found in up to 18.2% of these cases in some reviews.4 As in the cases reported by Magro et al.,25 eccrine coil appeared necrotic in one of our cases, which was previously reported to highlight thrombosis involving also dermo-hypodermal vessels.26
The results of immunohistochemistry as well as other techniques to detect the virus, such as qRT-PCR, ISH or RNAscope were frustrating in our skin series. Despite finding clearly positive results in COVID-19 lung with both tested antibodies, all the tested cutaneous cases were negative, as reported by other groups,16,27 and contrary to other articles.22 Discordant results have been reported with different antibodies,28 and even with different vials of the same antibody. Therefore, it is difficult to avoid considering any still underrecognized undefined cross reaction in some of the previously reported positive cases, or the deposition of cleaved spike protein. Liu et al. reported positive staining even in apparently non-involved skin from patients affected with SARS-CoV-2 infection thereby making direct viral involvement more difficult to prove.29
RT-PCR in our series of skin samples was negative as reported also in other articles in the literature,30,4 whereas 17 RT-PCR positive cases where found in other series.31
LimitationMany affected patients were only assessed clinically, without taking a biopsy. In addition, we faced some logistic problems as the transport of biological material between hospitals was restricted thus precluding the compilation of a larger series. We could not study our biopsies with electron microscopy as Garrido Ruiz et al.32
ConclusionsWe have presented the largest series of confirmed COVID-19 patients with histopathologically studied skin manifestations. Causality of viral infection was difficult to establish, as immunohistochemistry and other techniques to demonstrate the viral presence in the skin showed different results in our series compared with other published articles. Although direct viral presence was not found in cutaneous biopsies, vasculopathic and urticariform lesions were those more related epidemiologically to the viral infection.
In any case, as with all the emergent dermatological diseases, we want to highlight that clinico-pathological correlation is basic as SARS-CoV-2 seems to act as a trigger for many immunologically based dermatoses, and skin biopsy is paramount to exclude cutaneous manifestations independent of COVID-19.
FundingWe have received funding from Fundación para la Investigación Biomédica del Hospital Universitario de La Princesa for this study.
Conflict of interestsThe authors declare they have no conflict of interest.