Current guidelines call for baseline imaging only for very high-risk (T4b) primary cutaneous melanomas.
ObjectivesTo estimate the frequency of computed tomography (CT) at baseline staging of primary cutaneous melanoma and the diagnostic yield of CT; and to describe the types and frequencies of incidentaloma findings.
Material and methodsCross-sectional study of cutaneous melanoma cases (tumor classifications Tis to T4bN0M0) attended between 2008 and 2014 in a specialized melanoma unit. Reports of CT scans performed during baseline staging were reviewed to determine the frequency of positive scan results, incidentaloma findings, unit cost for detection of metastasis, and factors associated with the decision to order CT.
ResultsCT results were available for 310 of the 419 patients included (73.99%). The tumor classifications were as follows: Tis, 17; T1, 137; T2, 71; T3, 48; and T4, 37. The CT results were negative in 81.61%, and incidentalomas were found in 18.06%. Additional primary tumors were found in 2 patients (0.64%), and metastasis was identified in one patient (0.32%). The cost of finding the case of metastasis was €71,234.90. A T2 tumor classification (odds ratio [OR], 8.73) and age under 70years (OR, 3.53) were associated with greater likelihood of CT being ordered. Excision of the primary tumor in the melanoma unit (OR, 0.08) was associated with less likelihood of ordering CT.
ConclusionsThe results for this patient series support current recommendations restricting CT at baseline to cases where there is high risk of metastasis (stagesiiC-iii).
Las recomendaciones actuales de pruebas de imagen en la estadificación basal del paciente con melanoma cutáneo primario se limitan a los estadios tumorales de riesgo elevado (T4b).
ObjetivoEvaluar la frecuencia y el rendimiento de la tomografía computarizada (TC) para la estadificación basal del paciente con melanoma cutáneo primario y la tipología y la frecuencia de los incidentalomas identificados.
Material y métodosEstudio transversal sobre pacientes con melanoma cutáneo de estadio Tis-T4bN0M0 atendidos entre 2008 y 2014 en una Unidad de Melanoma. Se revisaron las TC realizadas como parte del estudio de estadificación basal para obtener la frecuencia de TC positiva, incidentalomas, coste unitario de la detección de metástasis y factores asociados a la realización de TC.
ResultadosSobre un total de 419 pacientes incluidos se realizó TC basal en el 73,99% de los pacientes (n=310TC. Tis=17, T1=137, T2=71, T3=48, T4=37), de las que el 81,61% fueron negativas y el 18,06% presentaron incidentalomas. En 2 pacientes (0,64%) se identificaron segundas neoplasias primarias y en un paciente, metástasis de melanoma (0,32%). El coste asociado a la identificación de metástasis fue de 71.234,90€/metástasis. El estadio T2 (OR=8,73) y la edad <70años (OR=3,53) se asociaron con mayor probabilidad de solicitud de TC; la exéresis del tumor primario en la Unidad de Melanoma (OR=0,08) se asoció con menor probabilidad de solicitud de TC.
ConclusionesLos resultados obtenidos en esta serie confirman las recomendaciones actuales que restringen la indicación de la TC de estadificación basal a los escenarios de alto riesgo de enfermedad metastásica (estadiosiiC-iii).
Epidemiological studies have shown an increase in the incidence of cutaneous melanoma (CM) during recent decades.1 However, most patients are diagnosed with tumors with a low Breslow thickness and, therefore, a more favorable initial prognosis.2
In the extension study of patients with CM, selective sentinel node biopsy (SSNB) is the gold standard for node staging in cases of stage≥T1b tumors. With respect to identification of distant metastasis, current guidelines limit their recommendation for advanced imaging tests (computed tomography [CT], magnetic resonance imaging [MRI], and positron emission tomography with CT [PET-CT]) to patients with stage T4 tumors.3–6 However, despite their low yield in baseline staging of asymptomatic patients with stage T1-T3 tumors, these imaging tests continue to be used.7,8
The objective of the present study was to evaluate the frequency and yield of CT for baseline staging in patients with melanoma at the Melanoma Unit of Hospital Universitario Virgen Macarena (UM-HUVM), Seville, Spain and to analyze factors that were potentially associated with the request for this imaging test. The secondary objective was to describe the frequency and type of incidentalomas identified by CT during baseline staging.
Material and MethodsWe performed a cross-sectional study of patients with primary CM attended at UM-HUVM between 2008 and 2014. UM-HUVM is a reference unit for the treatment of patients from the catchment area of HUVM and from other areas and centers who are referred for completion of their staging study with SSNB. The inclusion criteria were as follows: diagnosis of primary CM between January 1, 2008 and December 31, 2014; tumor stage Tis-T4b; baseline staging CT scan performed within 30 days after diagnosis of primary CM and before SSNB (if indicated); absence of detectable regional node involvement in the physical examination (provisional N0); and no suspicion of distant metastasis after taking the clinical history and performing the physical examination (provisional M0). The images included in the study were CT scans of at least the chest and abdomen in patients with CM on the trunk and extremities and CT scans of the head, neck, chest, and abdomen in patients with primary CM on the head and neck.9
The variables recorded for each of the patients included were as follows: age (in years and as a dichotomous variable ≤70years and >70years), sex, origin (UM-HUVM vs external center), Breslow thickness, tumor stage, result of CT scan (negative, positive for metastasis of melanoma, incidentaloma), type of incidentaloma (potentially relevant, not relevant), and location of incidentaloma. In accordance with accepted definitions, incidentaloma was defined as any casual finding with the appearance of a tumor on the baseline staging CT scan in the absence of specific clinical signs or symptoms. We did not record radiological findings associated with skeletal abnormalities that were not suggestive of metastasis (eg, osteoporosis and arthrosis), vascular abnormalities (eg, aneurysm and calcifications), or variations in normal anatomy.
Incidentalomas were considered potentially relevant when additional tests were performed to rule out or confirm their type (radiological follow-up, PET-CT, endoscopy, puncture for cytology, and biopsy). Incidentalomas were not considered relevant in cases where they did not lead to changes in approach or in the performance of additional tests. Data on the study variables were obtained from the melanoma registry of HUVM, the electronic clinical history, and the radiological information system.
The findings measured were as follows: frequency of CT, frequency of positive CT findings for metastasis, frequency of incidentaloma, frequency of relevant CT findings (positive for metastasis and second primary tumors), frequency of avoidable additional tests (nonrelevant incidentalomas), yield of CT, and unit cost of the detection of metastasis. The yield of CT was measured as the ratio of positive CT results for metastasis to the total number of CT scans performed. The calculation of the cost of detecting metastasis included €229.79 for each scan according to the public price list for services provided by the Andalusian Health Service.10
The statistical analysis was performed using XLSTAT 2014 for Mac (v.4.05 Addinsoft SARL). The differences between the variables were analyzed using a univariate model based on the chi-square test for independent qualitative variables and the t test for independent quantitative variables. The study was completed with a post hoc analysis based on a binary logistic regression model to calculate the odds ratio (OR) of performing a CT scan according to the independent variables categorized as dichotomous variables (dummy variables). Statistical significance was set at P<.05 (2-tailed).
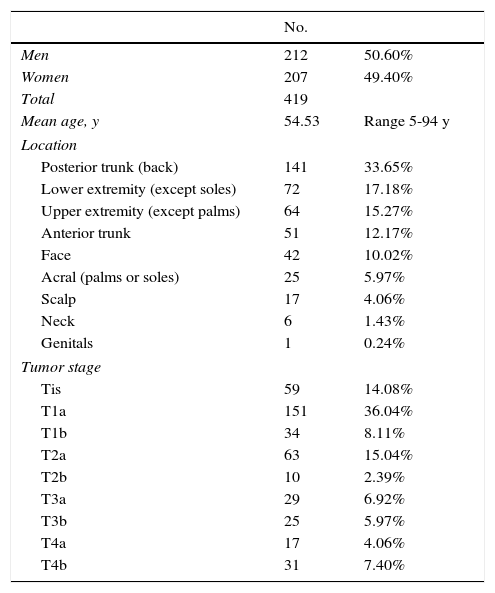
ResultsThe population assessed during the study period comprised 419 patients with primary CM, of whom 310 (73.99%) underwent CT for baseline staging. The baseline characteristics of the patients are shown in Table 1. The mean age of patients who underwent baseline CT was significantly lower than that of patients who did not (52.82 vs 59.39years, P<.001). No significant differences were observed for sex or performance of CT (51.29% men vs 48.71% women, P=.63).
Baseline Characteristics of Study Patients.
| No. | ||
|---|---|---|
| Men | 212 | 50.60% |
| Women | 207 | 49.40% |
| Total | 419 | |
| Mean age, y | 54.53 | Range 5-94 y |
| Location | ||
| Posterior trunk (back) | 141 | 33.65% |
| Lower extremity (except soles) | 72 | 17.18% |
| Upper extremity (except palms) | 64 | 15.27% |
| Anterior trunk | 51 | 12.17% |
| Face | 42 | 10.02% |
| Acral (palms or soles) | 25 | 5.97% |
| Scalp | 17 | 4.06% |
| Neck | 6 | 1.43% |
| Genitals | 1 | 0.24% |
| Tumor stage | ||
| Tis | 59 | 14.08% |
| T1a | 151 | 36.04% |
| T1b | 34 | 8.11% |
| T2a | 63 | 15.04% |
| T2b | 10 | 2.39% |
| T3a | 29 | 6.92% |
| T3b | 25 | 5.97% |
| T4a | 17 | 4.06% |
| T4b | 31 | 7.40% |
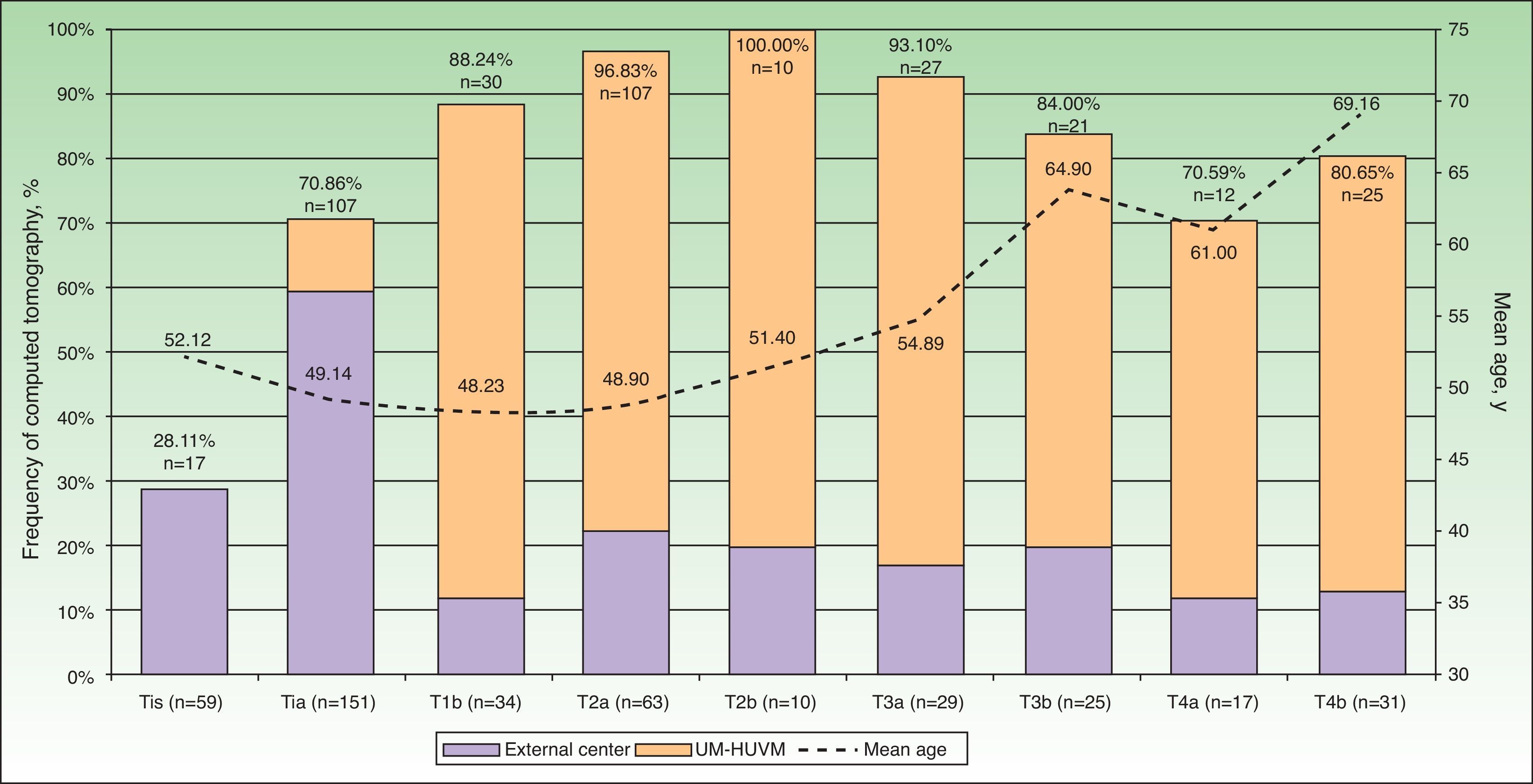
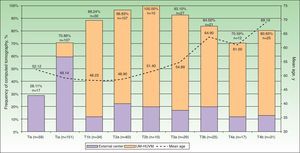
The frequency and absolute number of CT scans performed for each tumor stage and age are shown in Figure 1. CT was performed for the baseline extension study in 107 of the 151 patients with stage T1a disease (70.86%). The scan was indicated and performed at an external center in 90 cases (84.11%) before the patient was referred. In 17 cases (15.89%), the CT was ordered by the UM-HUVM.
Frequency of CT at baseline staging for each tumor stage (Tis-T4b). The percentages in the bars correspond to the frequency of CT and n to the number of CTs performed. The line shows the mean age of the study patients according to the progress of the initial tumor stage. The highest mean ages coincide with a decreasing frequency of CT. CT indicates computed tomography; Tis, melanoma in situ; UM-HUVM, Unidad de Melanoma, Hospital Universitario Virgen Macarena.
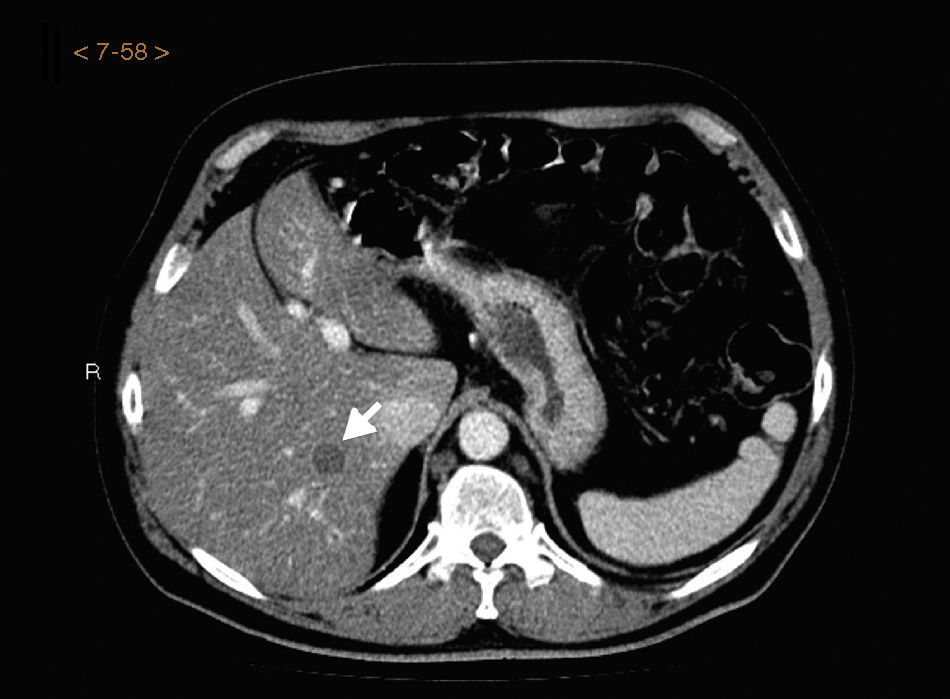
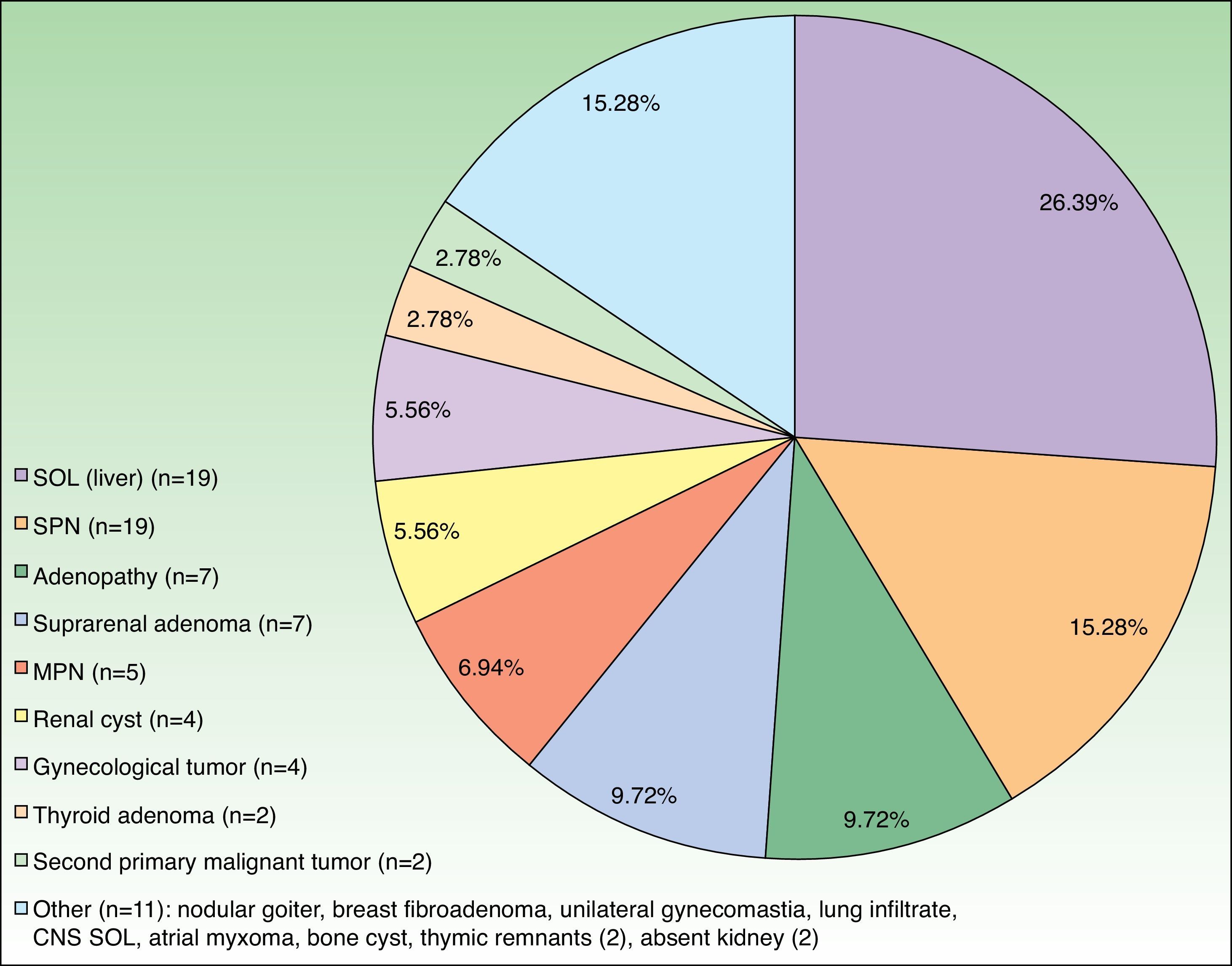
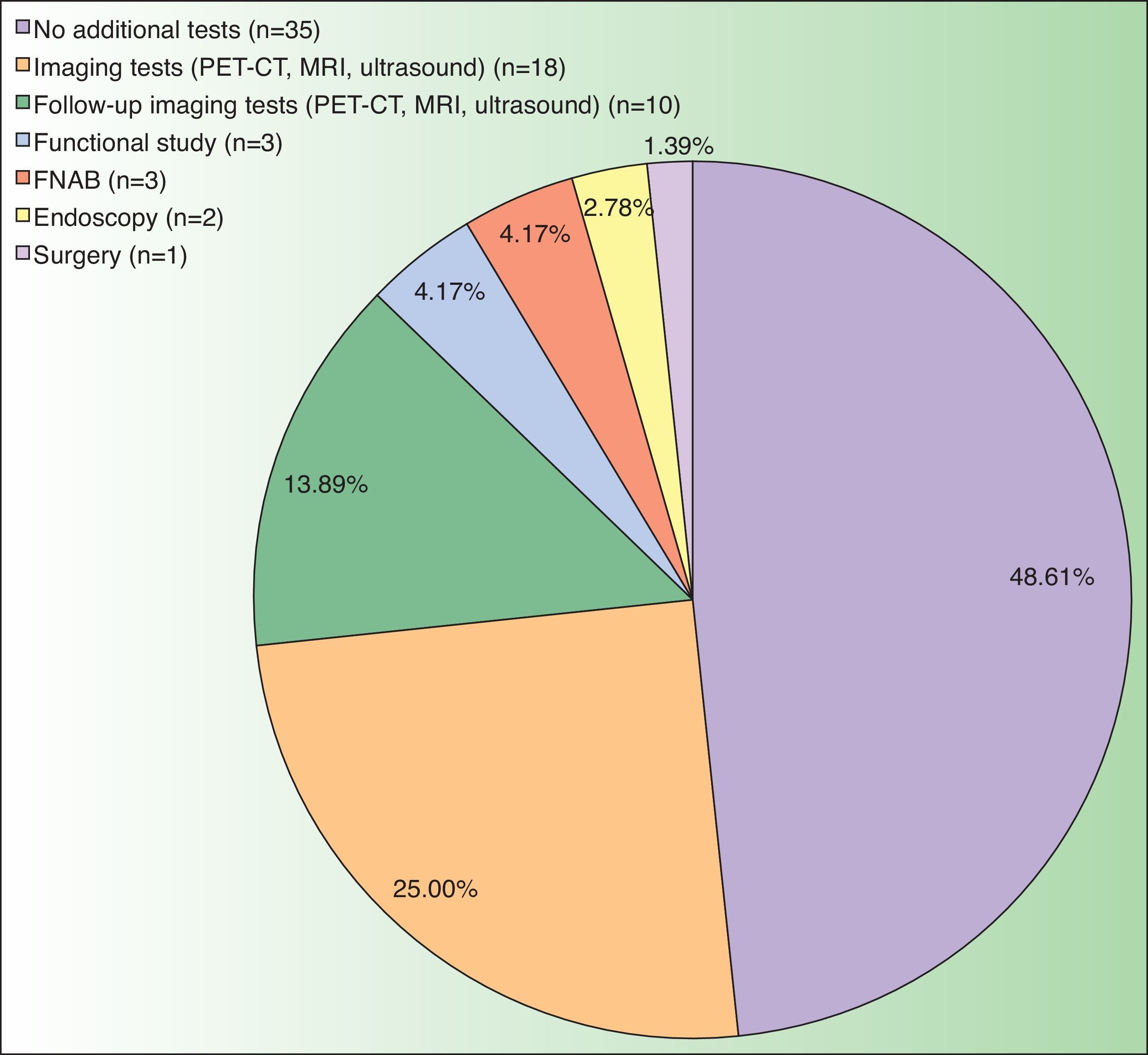
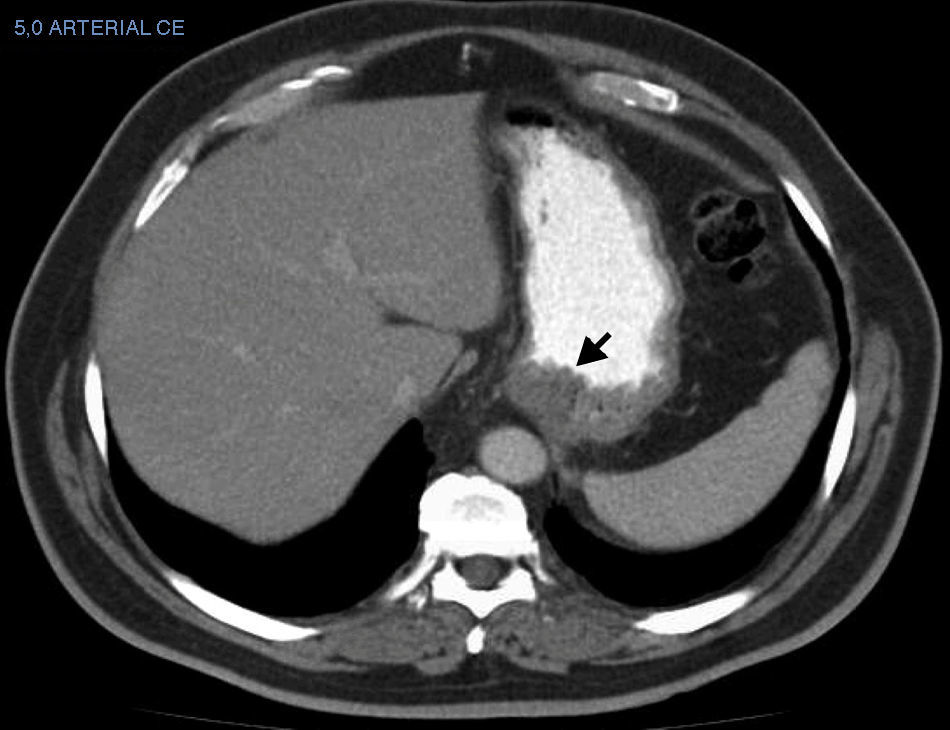
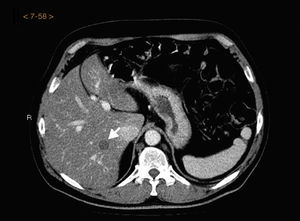
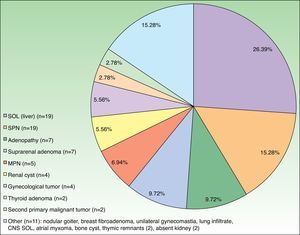
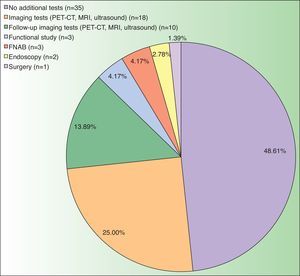
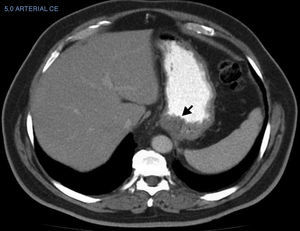
The CT result was negative in 253 cases (81.61%). A total of 72 incidentalomas were identified in 56 scans (18.06%). One scan (0.32%) performed on a patient with a stage T3a CM revealed an image that was suggestive of isolated liver metastasis, which was later confirmed after resection (Fig. 2). The most commonly detected incidentalomas were space-occupying lesions in the liver (19[26.39%]), followed by solitary pulmonary nodules (11[15.28%]) (Fig. 3). A total of 35 incidentalomas (48.61%) were classified as not relevant, whereas 37 (51.39%) were studied further (Fig. 4). In 2 cases (0.64%), the incidentalomas were adenocarcinoma of the stomach and lung carcinoma (Fig. 5). Three of the CT scans (0.97%) were relevant in oncological terms (1 patient with metastasis of melanoma and 2 patients with a second primary tumor).
The yield ratio of CT was 1 metastasis detected for every 310 scans performed. This ratio represents an associated cost of €71234.90 for each metastasis detected. If we assume that CT is performed on patients with stage T4b disease according to international recommendations, then the yield of CT in this series is 1:25 and the unit cost of metastasis is €5744.75.
The multivariate analysis showed that the variables associated with a greater likelihood of CT were stage T2 disease (OR=8.73; 95%CI, 1.77-43.21; P=.008) and age less than 70years (OR=3.53; 95%CI, 1.76-7.07; P=.001). Resection of the primary tumor with a request for an extension study in UM-HUVM (OR=0.08; 95%CI, 0.04-0.15; P=.001) was associated with a lower likelihood of CT than resection of the primary tumor at an external center.
DiscussionThe frequency of orders for CT in the series we report was higher than expected. Similar studies on patients with nonmetastatic primary CM report a frequency of advanced imaging tests (CT, PET-CT, and MRI) of 12.2%7 and 14.0%.8 The frequency of CT was 5.9%7 and 16.1%,8 and the frequency of PET-CT was 7.2%7 and 4.9%.8
The excessively frequent indication in the present study was noteworthy in patients with stage T1a tumors (70.86%) and even stage Tis tumors (28.81%). This deviation from guidelines mainly affected patients referred from external centers after resection of the primary tumor. In this sense, mention must be made of the greater frequency of T1a tumors in patients referred from external centers (61.59%) with respect to those who were attended initially and had their tumor removed in UM-HUVM (38.41%). Furthermore, in the case of patients attended at private centers, 25 patients with stage T1a tumors (100%) and 11 patients with melanoma in situ (84.61%) underwent baseline staging with CT in the absence of evidence in favor of this approach.
The decrease in the indication for CT in patients with stage T1a tumors in external centers was discreet, from 100% in 2008 (n=9scans) to 88.89% in 2014 (n=16scans). In any case, as the reference center, UM-HUVM is responsible for continuing to try to harmonize protocols between the collaborating centers.
The frequency of requests for CT in patients with stage T1b tumors (88.24%) is associated with the previously applied recommendation to perform CT in candidates for SSNB.9 Since this recommendation is not supported in the most recent guidelines, the trend since 2011 has been to perform ultrasound of lymph nodes as the only essential test before SSNB.
PET-CT was performed as the baseline extension study in 14 patients (3.34%), all of whose tumors were stage T3b or higher. Excluding the cases of melanoma in situ (n=59) would result in a frequency of orders for PET-CT of 5.58%, which is similar to that reported in the studies cited above.7,8 PET-CT is included in the guidelines of UM-HUVM and has therefore been funded since 2009 for the baseline extension study in patients whose primary tumor is stage T4. This guideline was revised at UM-HUVM in 2011 to include patients with stage T3b disease owing to the similar survival rates of patients with stage T3b and stage T4a tumors (2009 version of the American Joint Committee on Cancer melanoma staging system).11
In the study by Wasif et al.,7 the OR for performing axial imaging studies was significantly higher in older patients (OR=1.21 in patients aged 71-80years vs patients aged 65-70years), male patients (OR=0.86 in women vs men), and patients with advanced tumor stages (OR=2.66 T4 vs T1). These results are similar to those reported by Haddad et al.8 Performance of CT was also affected by age and tumor stage. However, our experience at UM-HUVM shows that the lower probability of offering radical treatment to elderly patients limits interest in and orders for imaging studies, including CT (OR=3.53 age ≤70 vs >70years) (Fig. 1). As for tumor stage, the increase in orders for PET-CT in intermediate-risk and high-risk stages (T3 and T4) could explain why the stage most associated with an order for CT in this study was T2 (OR=7.60 T2 vs other stages).
In the present series, the yield of CT for identification of subclinical metastasis of melanoma was comparable to that observed in similar studies (0.32%, ratio 1:310). Haddad et al.8 found that 1.36% of imaging tests revealed findings that led to suspicion of metastasis of melanoma, although none of these findings was confirmed. None of the CT scans performed in the study by Wasif et al.7 revealed findings that led to suspicion of metastasis of melanoma. These observations reinforce the recommendations of current guidelines to restrict imaging tests to high-risk stages based on clinical findings.4
There are no studies on incidentalomas during staging of melanoma, probably because there is no reason for the frequency of incidentalomas in patients with melanoma (18.06% in the present series) to differ from that of the general population. However, the frequent space-occupying lesions observed in the liver (26.39%) and lungs (22.22%, solitary and multiple pulmonary nodules), which are common sites of metastatic melanoma, necessitate additional tests to rule out metastasis. With respect to second tumors, those identified in the present study (1 lung cancer in a 77-year-old smoker with stage T4b disease and a 60-year-old patient with melanoma in situ and gastric adenocarcinoma) do not allow us to make recommendations on the benefit of staging CT in the diagnosis of second tumors.
Overindication of imaging tests for staging of patients with low-risk melanoma is associated with unfavorable consequences. Although we did not analyze this aspect in the present study, a finding of incidentaloma is usually accompanied by increased anxiety and uncertainty during the process of diagnosis, even after the tumor has been confirmed as benign. In addition, identification of an incidentaloma can lead to further tests (51.39% in the present series) with an avoidable risk of morbidity (eg, surgical biopsy and endoscopy). It is also necessary to evaluate the economic cost of a diagnostic test in a setting with a low pretest probability, such as baseline staging of patients with CM. The opportunity cost of an investment of €71234.90 to identify a metastatic melanoma could be redirected towards more efficient strategies. Mention must be made of redirection of investment toward improved accessibility to new treatments for metastatic melanoma or toward intensification of imaging tests in high-risk patients.12
The present study is subject to limitations, namely, its retrospective design and the fact that the cost per procedure was based on an official public price list from 2004. Nevertheless, the price list remains in force in the study setting.10
Consistent with current recommendations, the dermatologist caring for patients with melanoma should consider restricting CT to cases involving a high risk of metastasis (stagesIIC-III). The hypothetical benefit in terms of greater patient safety and reduced uncertainty is clearly offset by the low diagnostic yield, the potential anxiety associated with the finding of an incidentaloma, the possibility of comorbidity associated with additional tests, and the high economic cost of identifying a metastasis.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study
Confidentiality of dataThe authors declare that no private patient data appear in this article.
Right to privacy and informed consentThe authors obtained informed consent from the patients and/or subjects referred to in this article. This document is held by the corresponding author
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ferrándiz L, Silla-Prósper M, García-de-la-Oliva A, Mendonça FM, Ojeda-Vila T, Moreno-Ramírez D. Rendimiento de la tomografía computarizada en la estadificación basal del paciente con melanoma. Actas Dermosifiliogr. 2016;107:55–61.