To the Editor:
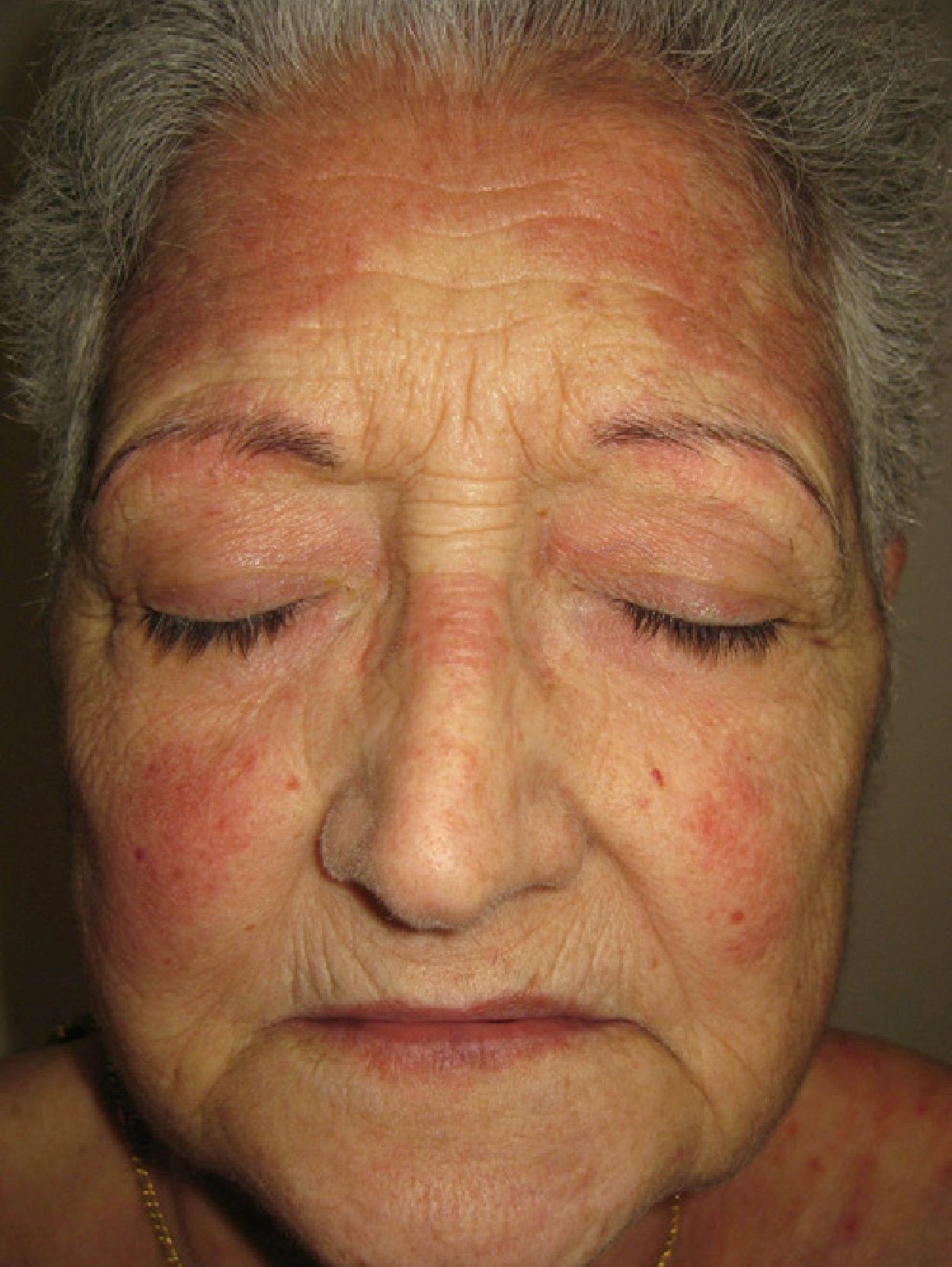
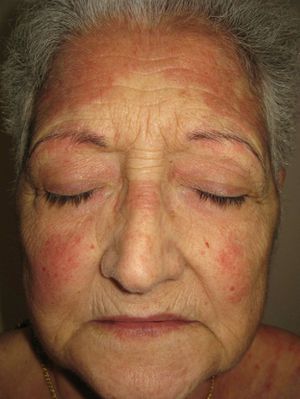
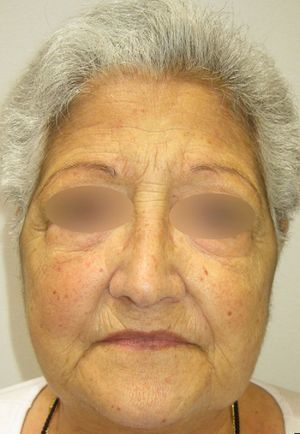
We report the case of a 67-year-old woman who presented with quinacrine-induced skin toxicity. At 32 years of age, the patient was diagnosed with systemic lupus erythematosus with both cutaneous and pleural manifestations, for which she was treated for many years with prednisone and chloroquine. Several years ago she was diagnosed with peripheral chorioretinal atrophy (retinitis pigmentosa) and chloroquine treatment was suspended. One year ago, upon reducing the prednisone dose from 30 to 15mg/day, the patient experienced an outbreak of erythematous lesions that affected the face (Fig. 1), trunk, and extremities. A skin biopsy revealed orthokeratotic hyperkeratosis, epidermal atrophy, vacuolar degeneration of the basal membrane, loss of skin appendages, and chronic inflammatory interstitial and perivascular infiltrate. Analyses revealed an anti-nuclear antibody titre of 1/160 with a speckled pattern, anti-Ro/SS-A antibodies (124 U/mL), and anti-DNA antibodies (5 U/mL). Other examinations revealed no relevant findings and the patient was diagnosed with an outbreak of cutaneous lupus erythematosus. Topical tacrolimus (0.1%) was administered, but was not tolerated, and after analyzing serum levels of thiopurine methyltransferase the patient was treated with azathioprine at 50mg/day. After 4 months, azathioprine treatment was stopped due to general malaise and abnormal test results: erythrocyte sedimentation rate, 86mm/h; aspartate aminotransferase, 41 U/L; alanine aminotransferase, 59 U/L; gamma-glutamyl transferase, 1006 U/L; alkaline phosphatase, 240 U/L. The cutaneous lupus erythematosus persisted with no improvement, and as the patient did not wish to take more immunosuppressants, quinacrine for compassionate use was obtained from the hospital pharmacy service. After providing informed consent the patient began treatment with quinacrine (100mg/day) and prednisone (50mg/day). Two months later the lupus erythematosus lesions had completely resolved with no residual scarring, allowing a reduction in the dose of prednisone.
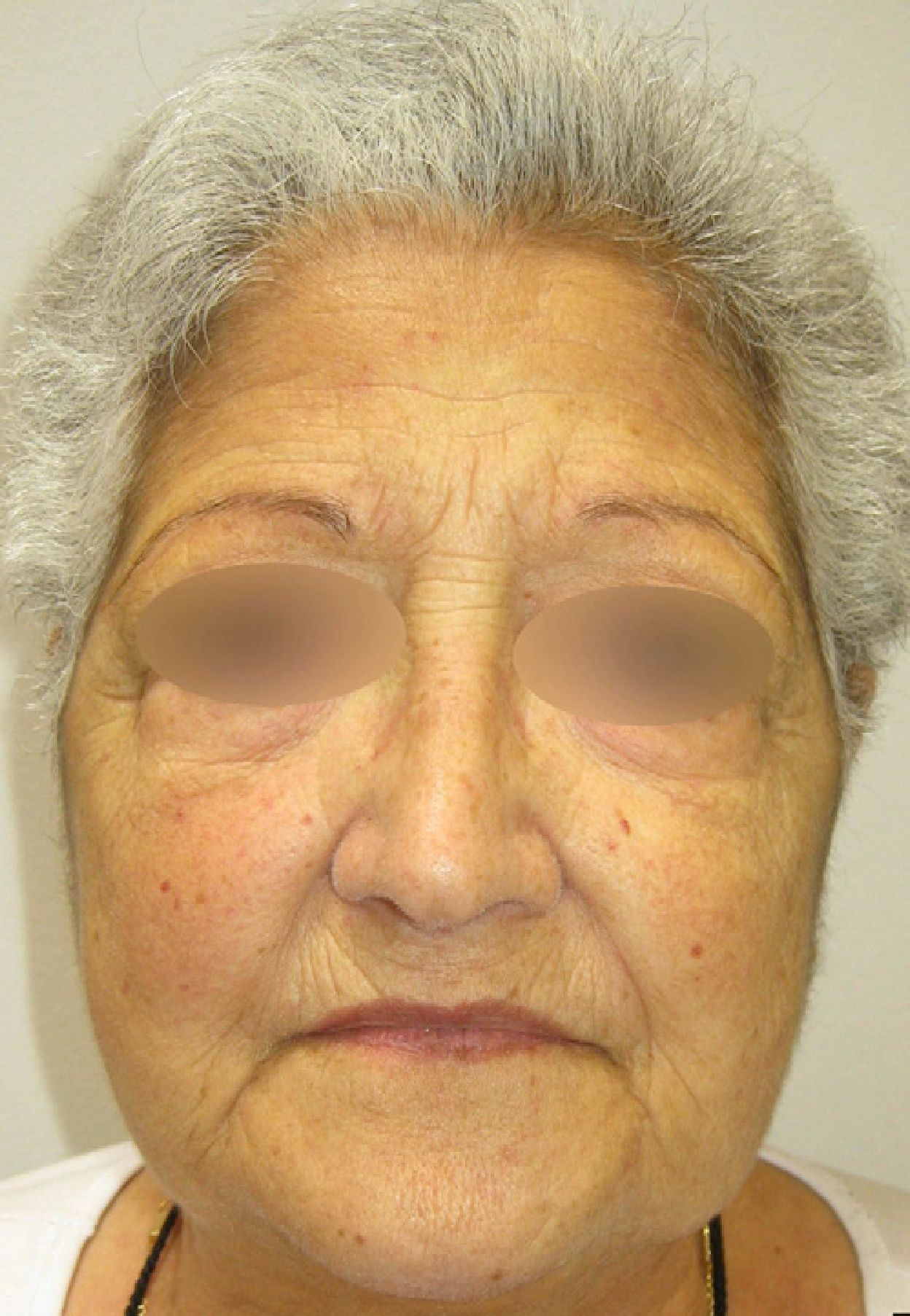
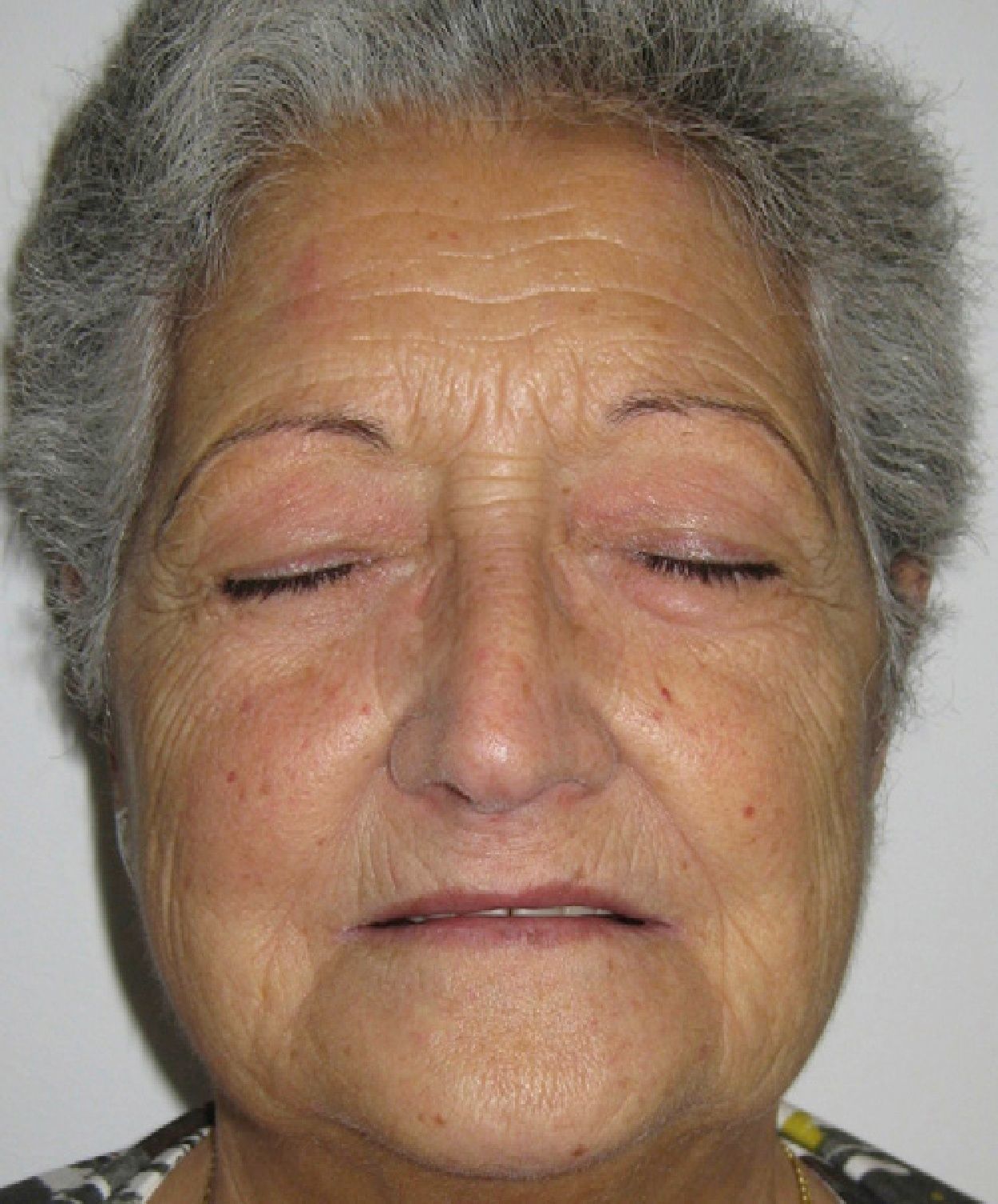
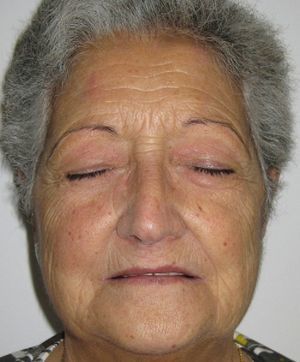
Five months after starting quinacrine treatment the prednisone dose had been reduced to 15mg/day, but the patient began to experience yellow discoloration of the skin and sclera (Fig. 2). As liver function and bilirubin levels were normal, quinacrine treatment was suspended. In the following months the skin regained its normal color and methotrexate was administered to prevent lupus erythematosus relapse. Currently the patient remains free of lupus erythematosus lesions and is being treated with prednisone (2.5mg/day) and methotrexate (5mg/week) (Fig. 3).
Quinacrine, also known as atabrine or mepacrine, is a synthetic derivative of quinine that was introduced in 1930 to prevent and treat malaria. Since then it has also been used for the treatment of giardiasis, taeniasis, and lupus erythematosus.1,2 Its chemical structure differs from that of chloroquine and hydroxychloroquine due the presence of an additional benzene ring. It is less effective than other antimalarials, but has the advantage of not causing retinal toxicity. This compound is not commercially available in Spain, but can be acquired through hospital pharmacy services as a foreign medication or for compassionate use. The usual dose is 100mg/day, and adverse effects include yellow discoloration of mucocutaneous zones,3 lichenoid eruption, aplastic anemia, headache, gastrointestinal symptoms, psychosis, convulsions and worsening of psoriasis. As yellowing of the skin and the whites of the eyes is very common, patients should be advised of this side effect before starting treatment. The majority of patients tolerate this discoloration, which usually occurs during the first weeks of treatment and resolves completely when treatment is discontinued. While the cause of the discoloration remains unknown, it is not due to hyperbilirubinemia and is related to the cumulative dose. In a series of 8 patients with cutaneous lupus erythematosus who were treated with quinacrine, the majority responded favorably, although 50% developed yellow skin discoloration.4
In summary, despite its multiple adverse effects, quinacrine is a useful drug for the treatment of patients with cutaneous lupus erythematosus with retinopathy. In our patient, quinacrine helped to resolve cutaneous lupus erythematosus and made possible a reduction in the dose of prednisone, although skin toxicity eventually led to withdrawal of the treatment.
Please cite this article: Vidal D, et al. Coloración amarillenta inducida por quinacrina en una paciente con lupus eritematoso cutáneo. Actas Dermosifiliogr.2013;104:89-90.