The Itch Severity Scale (ISS) facilitates objective assessment of the intensity of pruritus. The aim of this study was to validate a Spanish version of the ISS in patients with atopic dermatitis.
Material and methodsA prospective epidemiological study was undertaken in patients diagnosed with atopic dermatitis at least 1 year previously and a control group without the disease. Patients with atopic dermatitis were stratified according to the status of the lesions (active or inactive) and questionnaires were completed at baseline and 3-month and 6-month follow-up. Data were collected on sociodemographic variables relating to atopic dermatitis (including the modified Eczema Area and Severity Index [mEASI]), concomitant disease, and patient measures such as ISS, Dermatology Life Quality Index (DLQI), and Children's DLQI (cDLQI).
ResultsA total of 207 children (2-17 years) were included: 56 control subjects, 103 patients with active lesions, and 48 with inactive lesions. The mean (SD) age of the participants in this age group was 8.1 (4.0) years. A total of 261 adults (≥ 18 years) were included: 89 control subjects, 124 patients with active lesions, and 48 with inactive lesions. The mean age of the adult participants was 32.3 (13.4) years. A response rate of > 80% was obtained on the pediatric ISS (feasibility) and the responses correlated with the mEASI and cDLQI at baseline (P<.001) as an indicator of validity. An effect size of 0.988 was observed (sensitivity to change) along with a Chronbach α of 0.840 (internal consistency). A response rate of >95% was obtained on the adult ISS (feasibility) and the responses correlated with the mEASI and DLQI at baseline (P<.001) as an indicator of validity. An effect size of 1.0 was observed (sensitivity to change) along with a Chronbach α of 0.825 (internal consistency).
ConclusionsThe Spanish version of the ISS is feasible, valid, sensitive to change, and displays good reliability based on internal consistency in both children and adults.
El cuestionario Itch Severity Scale (ISS) permite evaluar objetivamente la intensidad del prurito. El presente estudio pretende validar la versión española del cuestionario ISS en pacientes con dermatitis atópica (DA).
Materiales y métodosEstudio epidemiológico prospectivo, incluyendo pacientes con DA de más de un año de evolución y un grupo control sin DA. Los pacientes con DA se estratificaron según actividad de las lesiones (activas/inactivas), realizando una visita basal y dos de seguimiento (3 y 6 meses). Se recogieron variables sociodemográficas, relacionadas con la DA (incluyendo el modified Eczema Area and Severity Index o mEASI), enfermedades concomitantes y medidas del paciente como ISS, Dermatology Life Quality Index (DLQI) o Children's DLQI (cDLQI).
ResultadosSe incluyeron 207 pacientes pediátricos (2-17 años): 56 controles, 103 con lesiones activas y 48 inactivas, con una edad media (DE) de 8,1(4,0) años. Los adultos fueron 261 pacientes (≥ 18 años): 89 controles, 124 con lesiones activas y 48 inactivas, con una edad media (DE) de 32,3 (13,4) años.
ISS pediátricos: tasa de respuesta > 80% (factibilidad), correlacionándose con mEASI y cDLQI en visita basal (p<0,001) (validez), effect size de 0,988 (sensibilidad al cambio) y alpha de Cronbach de 0,840 (consistencia interna).
ISS adultos: tasa de respuesta fue > 95% (factibilidad), correlacionándose con mEASI y DLQI en visita basal (p<0,001) (validez), effect size de 1,0 (sensibilidad al cambio), alpha de Cronbach de 0,825 (consistencia interna).
ConclusionesLa versión española del ISS se mostró factible, válida, sensible al cambio y fiable en términos de consistencia interna, tanto en niños como en adultos.
Atopic dermatitis (AD) is a chronic inflammatory skin disease that causes pruritus, xerosis, and scaling. Its clinical course is marked by periods of exacerbation and remission.1,2 Little is known about the prevalence of AD in Spain. Although the International Study of Asthma and Allergies in Childhood reported that 10.3% of adolescents had had the condition at some point in their lives,3 that estimate was based on a questionnaire survey that had not been validated for use in the Spanish population The overall prevalence in adults has been estimated to be between 1% and 3%.4
AD and the pruritus it causes have a negative impact on health-related quality of life (HRQOL).5–8 Furthermore, pruritus, whether in the context of AD or not, can itself be a very debilitating symptom, leading to psychological problems and sleep disorders.9,10
The Itch Severity Scale (ISS), a recently developed and validated instrument for quantifying the intensity of pruritus and its effect, has been validated in patients with psoriasis.11 The ISS can also be used to assess the effectiveness of treatment and compare population groups.
Given the usefulness that such an instrument might have in clinical practice or research in Spain, the Spanish study group on Itch Severity in Patients With Atopic Dermatitis (PSEDA, the Spanish acronym) sought to produce a culturally adapted version of the ISS for use in both children and adults with AD. We present an analysis of the measurement properties of the Spanish version of the scale.
Material and MethodsThis naturalistic, prospective, epidemiologic, multicenter study was carried out in Spain between October 2007 and November 2008. A total of 115 dermatologists from hospitals all over the country participated.
The dermatologists selected subjects for 3 study groups: patients with AD who had clinically unstable or active lesions, patients with AD who had stable or inactive lesions, and a control group of patients who had benign skin tumors. Enrolled patients had been diagnosed at least 1 year earlier with AD (according to the criteria of Hanifin and Rajka12) or with a benign tumor. All pediatric patients were older than 2 years and younger than 18 years of age. Adults were aged 18 years or older. Patients with AD were seen at baseline and at 3 and 6 months; patients with tumors (control group) were seen only at baseline. Written informed consent was obtained from all patients (or from the parent or legal guardian of the children). The clinical research ethics committee of Hospital Universitario de La Princesa in Madrid approved the study.
The baseline visit was used to record sociodemographic variables (age, sex and education, and in adults, occupational status) and clinical variables related to AD (time since onset and severity), and concomitant diseases. Changes in AD severity were noted during both follow-up visits. Patient-centered information (self- or parent-reported) was collected at all visits by means of the ISS1 and the Dermatology Life Quality Index (DLQI),13 or the Children's Dermatology Life Quality Index (cDLQI) in the case of the pediatric AD group. The patient's own health assessment was also noted. The pediatric questionnaire was completed by the parent or legal guardian.
AD severity was assessed on the modified Eczema Area and Severity Index (mEASI),14 which gives an overall score between 0 (no disease) and 90 (maximum severity of disease).
The cDLQI and the DLQI are generic dermatologic HRQOL inventories. Each has 10 items and gives a score between 0 (minimal impact on HRQOL/good HRQOL) and 30 (maximum impact on HRQOL/poor HRQOL). The patients’ perception of their health status was assessed with a questionnaire with 7 categorical responses from “very good” to “very bad.”
The ISSa is a specific instrument for assessing and quantifying the intensity of pruritus. To develop the Spanish ISS, the original version11 was translated and culturally adapted for use in Spain following standard methods.14,15 In the definitive Spanish version finally developed (Appendix A), items are scored exactly as in the original version. The ISS contains 7 items and provides a global score between 0 (no itching) and 21 (very severe itching). Item 6, which refers to sexual desire and function is omitted from the pediatric version.
Statistical AnalysisDescriptive statistics for the baseline sociodemographic variables were compiled by patient group (patients with active AD lesions, patients with inactive AD lesions, and control patients with benign tumors) and by age group (children and adults). Continuous variables were described as mean (SD). Categorical variables were summarized by number of cases and percentage.
To validate the Spanish version of the ISS, we studied its measurement properties (feasibility, validity, sensitivity to change, and reliability). Feasibility was reflected by the number of test items and the percentage of items patients answered.
To analyze the structural validity and confirm the unidimensionality of the ISS, we carried out principal component analysis, calculated Pearson correlation coefficients, and performed analysis of variance (ANOVA). To estimate the correlation between the ISS score and the mEASI, cDLQI, and DLQI scores at baseline we used the Pearson or Spearman correlation coefficient, depending on the nature of the variable. Weak correlation was indicated by a coefficient of 0.3 or less, moderate correlation by a coefficient over 0.3 and less than 0.5, and strong correlation by a coefficient of 0.5 or more.
To analyze sensitivity to change, the change in ISS score from baseline to each follow-up visit in relation to changes in the patient's own perception of state of health at that time was subjected to repeated-measures ANOVA. The effect size was calculated for each category. Change was recorded as improvement (positive change in category), no change (same category), or worsening (negative change in category). We also calculated the minimal important difference (MID), defined as the difference in ISS score associated with the patients’ perception of an important change in health. For that purpose, we selected patients who showed improvement in 2 categories between the baseline and 3-month visits as well as a change in DLQI of 2 to 5 points in the same period.
Reliability was reflected by internal consistency (Cronbach α). Test-retest reliability was assessed based on scores recorded during the 3-month and 6-month follow-up visits, for patients whose condition remained stable. An intraclass correlation coefficient (ICC) of 0.7 or higher was considered to indicate acceptable reliability.
Statistics were compiled using the SAS software package, version 9.1 for Windows. In all statistical tests the level of significance was set at a value of P less than .05.
ResultsStudy Population CharacteristicsWe recruited 468 patients with AD or benign tumors detected at least 1 year earlier; 207 were pediatric patients (aged 2-17 years) and 207 were adults (≥18 years old). Among the pediatric participants, 103 and 48 had active and inactive AD lesions, respectively, and 56 had tumors (controls). Among the adult patients, 124 and 48 had active and inactive AD lesions, respectively, and 89 had tumors.
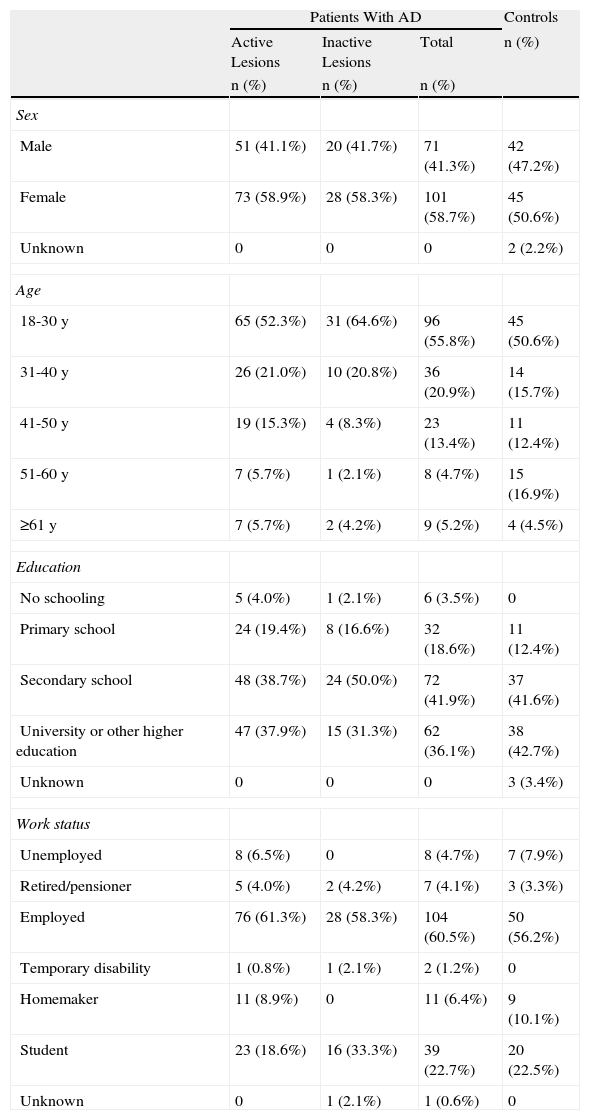
The mean age of children with AD was 8.1 (4.0) years, while the mean age of the pediatric control patients was 9.4 (4.5) years (P<.05). Table 1 shows the sociodemographic characteristics for all 3 pediatric groups. The adults with AD had a mean age of 32.3 (13.4) years, while the mean age for the adult controls was 35.2 (14.7) years (Table 2).
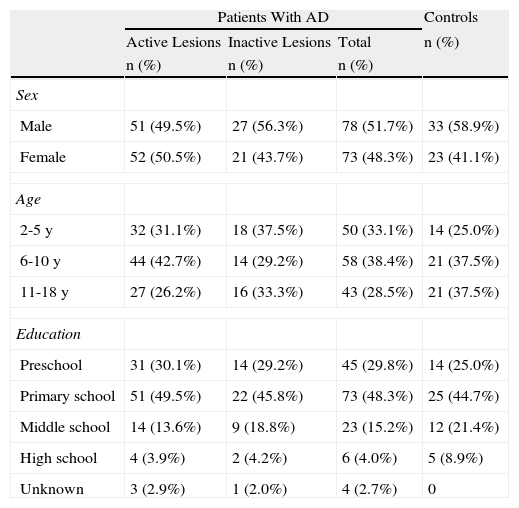
Sociodemographic Characteristics of the Pediatric Population by Study Group.
| Patients With AD | Controls | |||
| Active Lesions | Inactive Lesions | Total | n (%) | |
| n (%) | n (%) | n (%) | ||
| Sex | ||||
| Male | 51 (49.5%) | 27 (56.3%) | 78 (51.7%) | 33 (58.9%) |
| Female | 52 (50.5%) | 21 (43.7%) | 73 (48.3%) | 23 (41.1%) |
| Age | ||||
| 2-5 y | 32 (31.1%) | 18 (37.5%) | 50 (33.1%) | 14 (25.0%) |
| 6-10 y | 44 (42.7%) | 14 (29.2%) | 58 (38.4%) | 21 (37.5%) |
| 11-18 y | 27 (26.2%) | 16 (33.3%) | 43 (28.5%) | 21 (37.5%) |
| Education | ||||
| Preschool | 31 (30.1%) | 14 (29.2%) | 45 (29.8%) | 14 (25.0%) |
| Primary school | 51 (49.5%) | 22 (45.8%) | 73 (48.3%) | 25 (44.7%) |
| Middle school | 14 (13.6%) | 9 (18.8%) | 23 (15.2%) | 12 (21.4%) |
| High school | 4 (3.9%) | 2 (4.2%) | 6 (4.0%) | 5 (8.9%) |
| Unknown | 3 (2.9%) | 1 (2.0%) | 4 (2.7%) | 0 |
Sociodemographic Characteristics of the Adult Population by Study Group.
| Patients With AD | Controls | |||
| Active Lesions | Inactive Lesions | Total | n (%) | |
| n (%) | n (%) | n (%) | ||
| Sex | ||||
| Male | 51 (41.1%) | 20 (41.7%) | 71 (41.3%) | 42 (47.2%) |
| Female | 73 (58.9%) | 28 (58.3%) | 101 (58.7%) | 45 (50.6%) |
| Unknown | 0 | 0 | 0 | 2 (2.2%) |
| Age | ||||
| 18-30 y | 65 (52.3%) | 31 (64.6%) | 96 (55.8%) | 45 (50.6%) |
| 31-40 y | 26 (21.0%) | 10 (20.8%) | 36 (20.9%) | 14 (15.7%) |
| 41-50 y | 19 (15.3%) | 4 (8.3%) | 23 (13.4%) | 11 (12.4%) |
| 51-60 y | 7 (5.7%) | 1 (2.1%) | 8 (4.7%) | 15 (16.9%) |
| ≥61 y | 7 (5.7%) | 2 (4.2%) | 9 (5.2%) | 4 (4.5%) |
| Education | ||||
| No schooling | 5 (4.0%) | 1 (2.1%) | 6 (3.5%) | 0 |
| Primary school | 24 (19.4%) | 8 (16.6%) | 32 (18.6%) | 11 (12.4%) |
| Secondary school | 48 (38.7%) | 24 (50.0%) | 72 (41.9%) | 37 (41.6%) |
| University or other higher education | 47 (37.9%) | 15 (31.3%) | 62 (36.1%) | 38 (42.7%) |
| Unknown | 0 | 0 | 0 | 3 (3.4%) |
| Work status | ||||
| Unemployed | 8 (6.5%) | 0 | 8 (4.7%) | 7 (7.9%) |
| Retired/pensioner | 5 (4.0%) | 2 (4.2%) | 7 (4.1%) | 3 (3.3%) |
| Employed | 76 (61.3%) | 28 (58.3%) | 104 (60.5%) | 50 (56.2%) |
| Temporary disability | 1 (0.8%) | 1 (2.1%) | 2 (1.2%) | 0 |
| Homemaker | 11 (8.9%) | 0 | 11 (6.4%) | 9 (10.1%) |
| Student | 23 (18.6%) | 16 (33.3%) | 39 (22.7%) | 20 (22.5%) |
| Unknown | 0 | 1 (2.1%) | 1 (0.6%) | 0 |
Congenital melanocytic nevus (50.0%) and intradermal melanocytic nevus (53.9%) were the most common tumor types in the pediatric and adult control groups, respectively.
Concomitant diseases were noted in 43.7% of the pediatric patients with AD, whereas only 17.9% of the pediatric controls had comorbidity (P<.001). In the group of adults with AD, 57.6% had concomitant diseases, in contrast with 40.5% of the adult controls (P<.01).
The mean time elapsed since onset of disease was 5.0 (3.4) years for the children with AD and 2.8 (3.5) years for the pediatric control patients with benign tumors. For adults the mean time elapsed since onset was 14.9 (11.7) years for the patients with AD and 6.4 (10.4) years for the control patients with tumors.
The mEASI scores showed improvement for the children with AD during the study, as reflected by a mean reduction of 8.6 points (95% confidence interval [CI], 6.6-10.6 points) between baseline and the 3-month visit (P<.001) and a mean reduction of 12.3 points (95% CI, 9.9-14.8 points) at the 6-month visit. Improvement was perceived by 64.5% of the patients between the baseline and 3-month visit and by 78.7% at 6 months. The cDLQI showed a mean reduction of 3.9 points (95% CI, 2.8-5.0 points) from baseline at 3 months and of 5.5 points (95% CI, 4.0-7.0 points) at 6 months, indicating that disease had come to exercise less impact on HRQOL over the course of the study.
In adults, the mEASI demonstrated positive change in severity of AD during the study, as the mean score fell by a mean of 8.4 points (95% CI, 6.6-10.2 points) (P<.001) below baseline at the 3-month visit and by 11.0 points (95% CI, 8.9-13.2) at 6 months. The patients also reported sensing improvement in their health at both follow-up visits. At the 3-month visit, 67.0% declared their health had improved over baseline and at 6 months 74.0% held this opinion. A mean reduction of 3.2 points (95% CI, 2.5-3.9 points) was detected in the DLQI at 3 months. At 6 months, the reduction was a mean of 4.9 points (95% CI, 3.7-6.0 points) from baseline, indicating improved HRQOL.
ISS scores changed significantly at 3 months and at 6 months in comparison with baseline for both adults and children (P<.001 for both group comparisons). In the children, the ISS score fell by a mean of 2.5 points (95% CI, 1.9-3.0 points) at 3 months from baseline and by 3.5 points (95% CI, 2.8-4.1 points) at 6 months. In the adults the ISS score fell by a mean of 3.3 points (95% CI, 2.7-3.9 points) at 3 months and by 4.8 points (95% CI, 4.0-5.7 points) at 6 months, indicating that both children and adults experienced less pruritus.
Measurement Properties of the ISS in Pediatric PatientsFeasibilityThe parents or guardians of 67.6% of the children with AD responded to all survey items. The response rate exceeded 80.0% in all 3 pediatric groups, indicating that the questionnaire can be considered feasible.
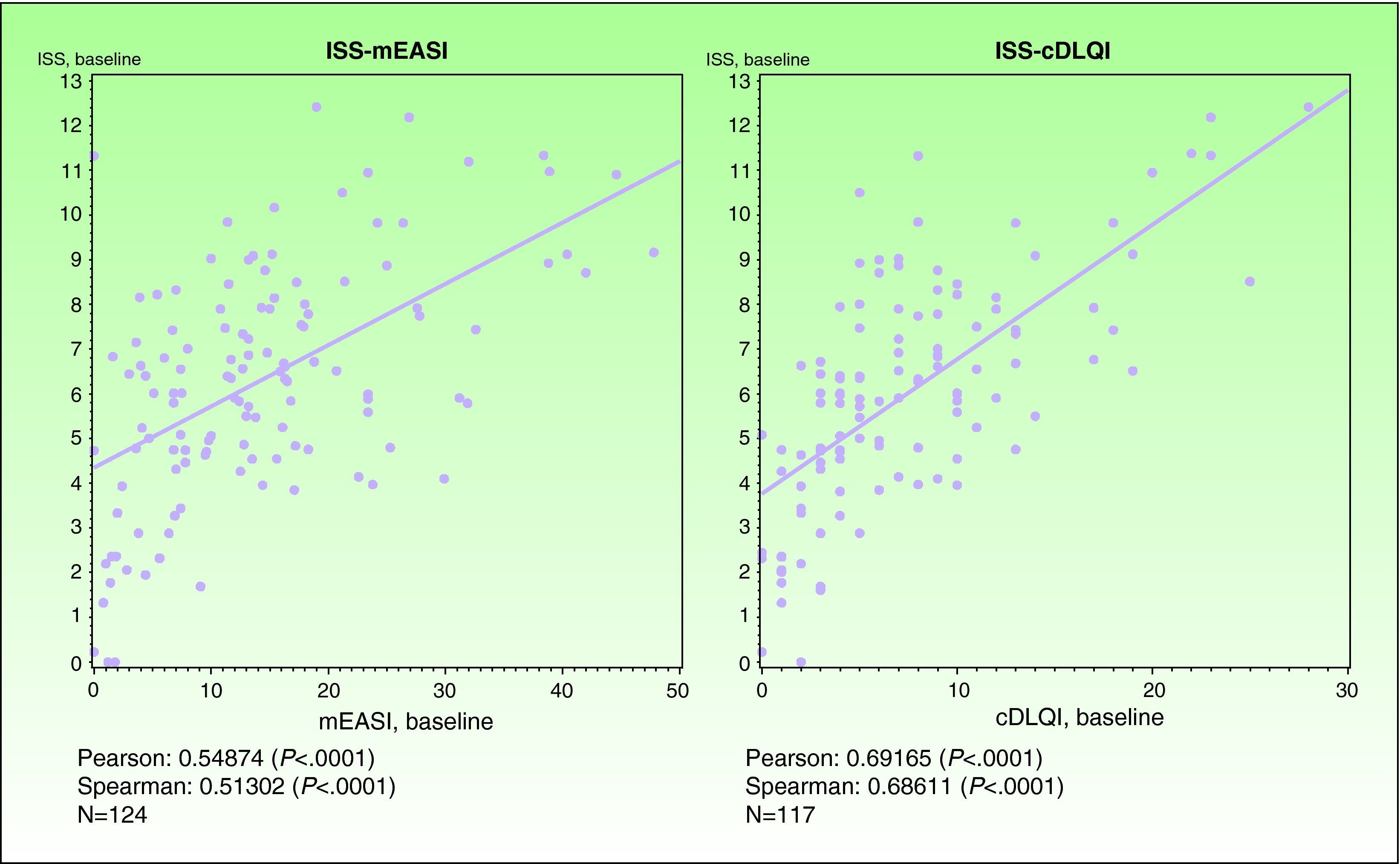
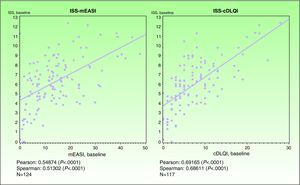
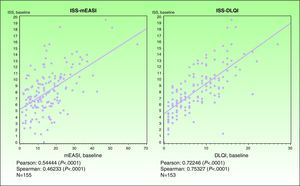
ValidityPrincipal component analysis showed that a single component explained 68.0% of the variance, confirming the unidimensionality of the Spanish ISS. Figure 1 shows the correlation between this ISS version and the mEASI and cDLQI at baseline. All correlations were positive, moderate, and statistically significant (P<.001).
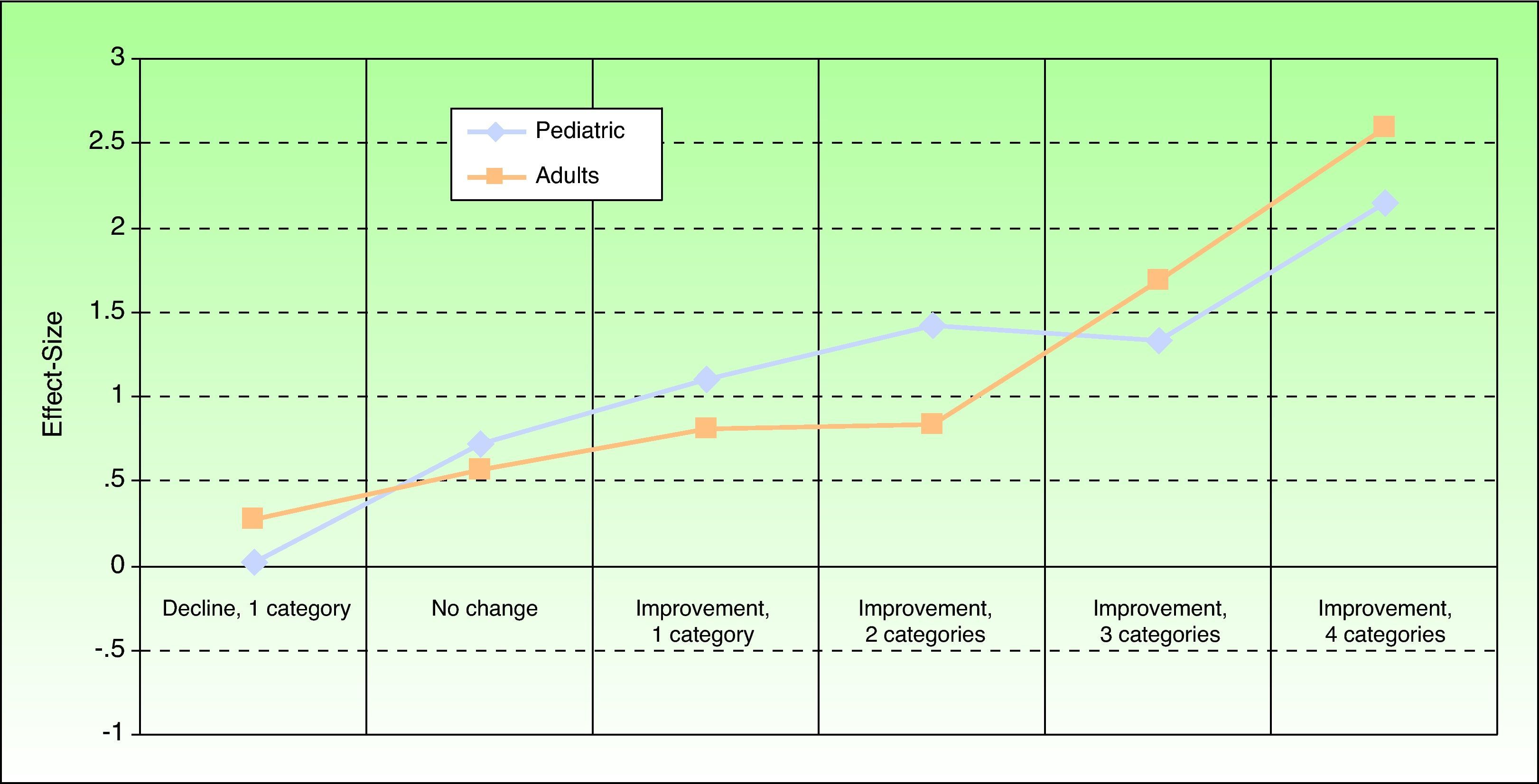
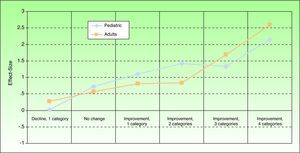
Sensitivity to ChangeThe effect size between baseline and 3 months, calculated to estimate sensitivity to change, was 0.988 (large effect size) (Fig. 2). The MID was 3 points (ie, a change of 3 points or more could be considered to reflect a significant change in HRQOL for the patient).
Effect size of the Spanish Itch Severity Scale in terms of self-reported state of health 3 months after baseline in children and adults. State of health was assessed by asking the patient for an opinion expressed in terms of the following possible answers: very good, fairly good, somewhat good, neither good nor bad, fairly bad, and very bad.
Good internal consistency was indicated by a Cronbach α of 0.840 (superior to threshold of 0.70). Test-retest reliability, estimated on the basis of scores at 3 and 6 months for patients with inactive lesions, was reflected by ICCs between 0.68 and 0.73. The ICCs were higher than 0.7 in 2 out of 3 previously defined stability criteria.
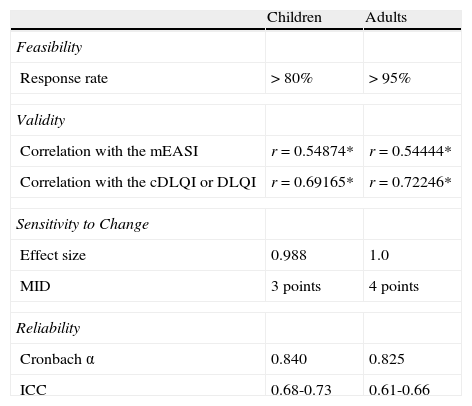
Measurement properties of the Spanish ISS in children are summarized in Table 3.
Measurement Properties of the Itch Severity Scale in Pediatric and Adult Populations.
| Children | Adults | |
| Feasibility | ||
| Response rate | > 80% | > 95% |
| Validity | ||
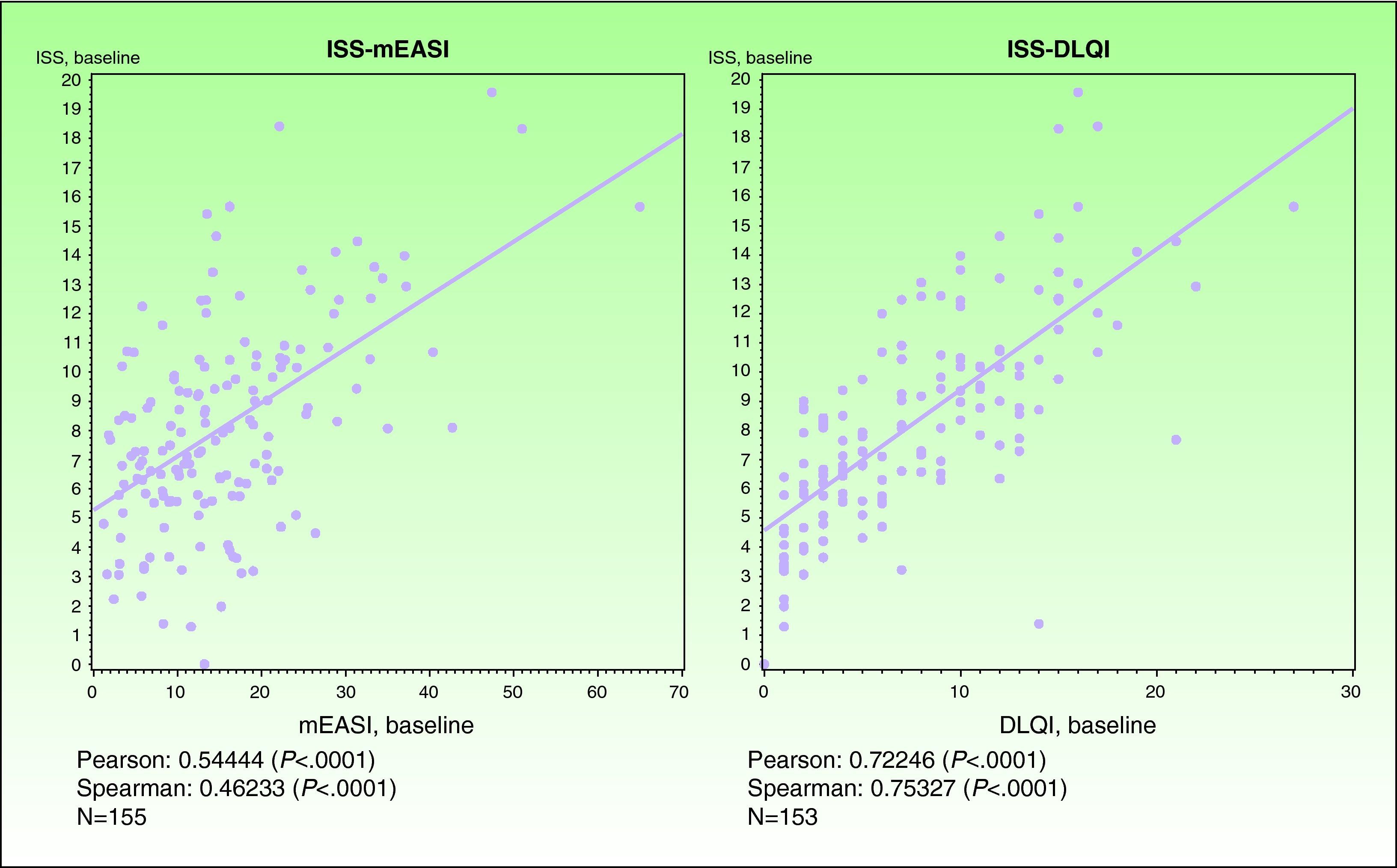
| Correlation with the mEASI | r = 0.54874* | r=0.54444* |
| Correlation with the cDLQI or DLQI | r=0.69165* | r=0.72246* |
| Sensitivity to Change | ||
| Effect size | 0.988 | 1.0 |
| MID | 3 points | 4 points |
| Reliability | ||
| Cronbach α | 0.840 | 0.825 |
| ICC | 0.68-0.73 | 0.61-0.66 |
* P<.0001. Abbreviations: cDLQI, Children's DLQI; DLQI, Dermatology Life Quality Index; ICC, intraclass correlation coefficient; MID, minimal important difference (between baseline and 3 mo); r, Pearson correlation coefficient.
All ISS items were answered by 89.0% of the patients with AD and by 77.5% of the control patients. The response rate was over 95% for all ISS items in both AD patient groups and over 90% in the control group. Given that over 80% of the items were answered, the Spanish ISS can be considered feasible.
ValidityPrincipal component analysis showed that a single component explained 61.0% of the variance, confirming the unidimensionality of the Spanish ISS. Figure 3 shows the correlation between this ISS version and the mEASI and DLQI scores at baseline. All correlations were positive, moderate, and statistically significant (P<.001).
Sensitivity to ChangeThe effect size, estimating sensitivity to change, was 1.0 (large effect size) between the baseline and 3-month visits (Fig. 2). The MID was between 3 and 4 points at 3 months and between 3.5 and 4.5 points at 6 months. Therefore a change of 4 points in the adult Spanish version of the ISS can be considered to reflect a significant change for the patient.
ReliabilityThe good internal consistency of the scale was indicated by a Cronbach α of 0.825 (higher than the stipulated value of 0.7). The test-retest reliability based on scores recorded for patients with inactive lesions at 3 and 6 months was reflected by an ICC between 0.61 and 0.66 (Table 3).
DiscussionThis study demonstrates that the Spanish version of the ISS is valid for use in both its adult and pediatric versions and that its measurement properties are similar to those of the original version.11 ISS scores correlated moderately with those of both the pediatric and adult versions of the DLQI.
No statistically significant differences in the sociodemographic characteristics of the AD patient and control groups were detected, indicating that the 3 groups were homogeneous. This similarity between the groups other than the finding of a greater frequency of diseases associated with AD than with benign tumors supports the robustness of the results we report.
The pediatric ISS has proven feasible for use in a Spanish population (response rate >80%) as well as valid; it is sensitive to change (large effect size) and has good internal consistency (Cronbach α more than 0.7). The properties of the pediatric version cannot be compared to those of the original ISS, which did not have a version for use in children.
The adult Spanish ISS also proved feasible and valid, with good sensitivity to change and reliability in terms of internal consistency. The correlations between ISS results on this version and scores on the mEASI and the DLQI at the baseline visit (ranging from 0.46 to 0.72) were higher than correlations found for the original version in relation to the DLQI (which ranged from 0.22-0.55).11 In both cases, however, the correlations were moderate, a finding that is not unusual in studies of the relation between clinical variables and HRQOL. A moderate correlation indicates that higher ISS scores correspond to patients with more serious AD whose state of health is poorer.
Given that itch intensity has considerable impact on a patient's health and perception of HRQOL,16 it seems reasonable for the ISS score to be related to the patient's own sense of health. In other words, the patient's report of having less itching will be consistent with feeling healthier.
The MIDs of the Spanish ISS allow us to assume that a patient will perceive improvement if there is a change of at least 4 points in the score. In the study to validate the original version of the ISS, the differences above which the instrument detected improvements was between 2.2 and 5.5 points.15
The levels of internal consistency of the Spanish version and the original version15 were also similar and in both cases over 0.7.
Test-retest reliability was found to lie between 0.61 and 0.66. Thus, this statistic did not reach or exceed the stipulated threshold of 0.70 for the Spanish version and fell below the test-retest reliability of the original. The failure to match this property is probably attributable to the difference in the timing of the first and second testing sessions in the 2 validation studies: retesting of the original version took place at 2 weeks, whereas retesting of the Spanish ISS took place after 3 months had passed. It is to be expected that test-retest reliability would be higher for the shorter period of time.
The subjective nature of itching might constitute a limitation of this study. As this symptom cannot be measured objectively, validity was assessed by comparing the Spanish ISS score to the same HRQOL questionnaire (the DLQI) that was used in validating the original version of the ISS. Other studies have found HRQOL to be affected in the presence of pruritus in patients with chronic AD,16 as well as in patients with other skin conditions, such as psoriasis17,18 or seborrheic dermatitis.19 Given these results, a dermatologist can use the Spanish ISS to monitor the degree or intensity of itching in patients as an indirect measure of the effectiveness of a treatment, to help in setting a treatment regimen for an individual patient.
ConclusionsThis study has demonstrated that, like the original version of the ISS, the Spanish instrument has good measurement properties in terms of feasibility, validity, sensitivity to change, and internal consistency. The Spanish ISS can be useful for evaluating the intensity of pruritus in patients with AD, whether children or adults, in routine practice.
FundingThis study was funded by Astellas Pharma, SA.
Conflict of InterestDr E. Daudén is or has been a member of advisory boards, has received consulting fees and research grants, has participated in clinical trials and received speaker's fees from the following companies: Abbott, Astellas, Biogen, Centocor Ortho Biotech Inc, Galderma, Glaxo, Janssen-Cilag, Leo Pharma, Merck-Serono, Pfizer, Novartis, Schering-Plough, Stiefel, Wyeth Pharmaceuticals, 3M, and Celgene.
Dr J. Sánchez-Pérez participates or has participated in clinical trials funded by the following pharmaceutical laboratories: Abbott, Astellas, Biogen, Galderma, Pfizer, Shering-Plough, and Wyeth Pharmaceuticals.
The authors express their thanks to all the researchers who participated in the PSEDA study.
Las siguientes frases se refieren a lo que usted puede sentir sobre el picor que padece.
Por favor, lea detenidamente cada una de las preguntas y sus instrucciones para responderlas. Señale aquella opción u opciones de respuesta que mejor describan lo que usted cree que le pasa.
No hay respuestas correctas o incorrectas. Simplemente estamos interesados en conocer lo que a usted le ocurre debido al picor que siente.
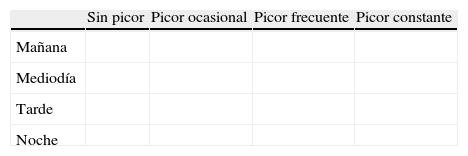
1. ¿Con qué frecuencia siente picor en cada momento del día? (por favor, marque con una «X» la casilla que corresponda a su respuesta)
2. ¿Hasta qué punto los siguientes términos describen el picor que siente? (por favor, marque con una «X» la casilla que corresponda a su respuesta)
3. Por favor, sombree las zonas donde suele sentir picor
4. Indique la intensidad del picor en cada uno de los siguientes casos (por favor, marque con una «X» la casilla que corresponda a su respuesta)
5. ¿Ha tenido cambios de humor debido al picor? (puede marcar más de una respuesta)
a. Ningún cambio.
b. Deprimido/a.
c. Más inquieto/a.
d. Dificultad para concentrarse.
e. Angustiado/a.
NOTA: La siguiente pregunta (pregunta n° 6) no deberá formularse en los pacientes menores de edad. No será tenida en cuenta para la puntuación final en este tipo de pacientes.
6. ¿Cómo le ha afectado el picor en lo siguiente? (por favor, marque con una «X» la casilla que corresponda a su respuesta)
7. Indique con qué frecuencia le ocurre cada una de las siguientes situaciones (por favor, marque con una «X»” la casilla que corresponda a su respuesta)
Si se quiere utilizar el cuestionario se debe solicitar autorización
al autor del cuestionario original.
The reader who wishes to use the questionnaire should seek permission from the original authors..
Please cite this article as: Daudén E, et al. Validación de la versión española de la escla de intensidad del pico (Cuestionario itch Severity Scale, ISS). Estudio PSEDA. Actas Dermosifiliogr. 2011;102:527-36.