Angiosarcoma is an aggressive tumor of endothelial origin that is associated with a poor prognosis. The effectiveness of new treatments such as bevacizumab is based on their ability to inhibit angiogenesis; specifically, bevacizumab acts on vascular endothelial growth factor (VEGF), which has been implicated in the pathogenesis of angiosarcoma.1,2
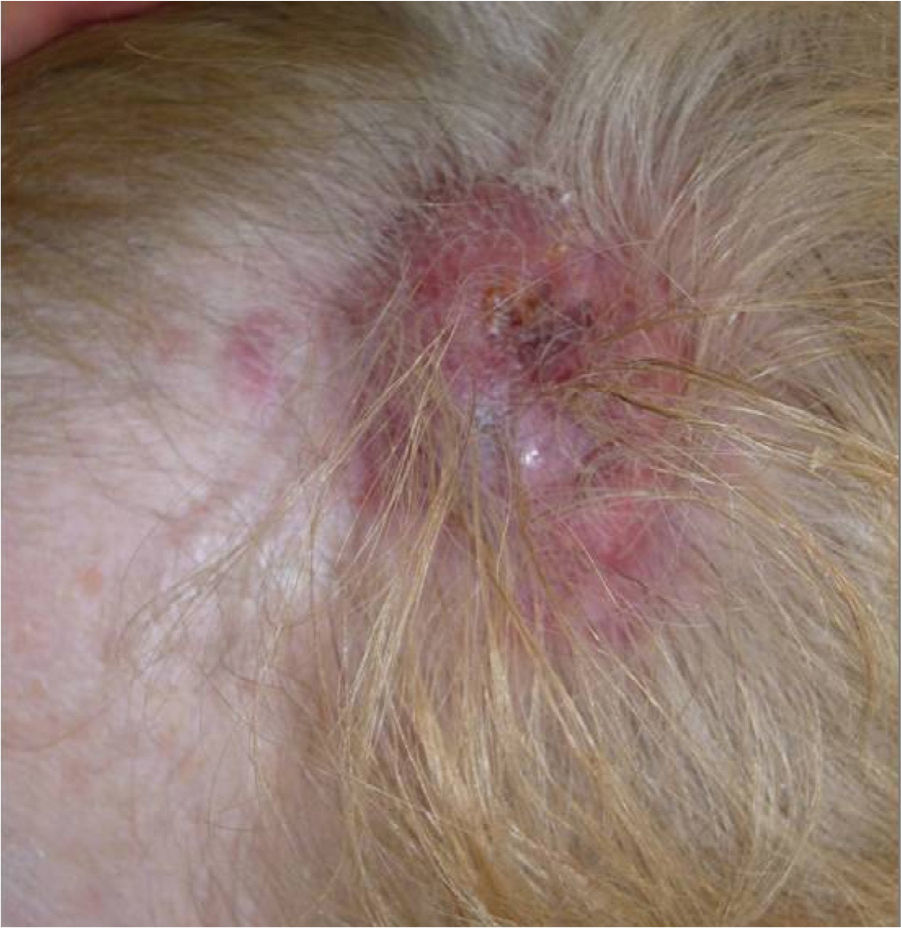
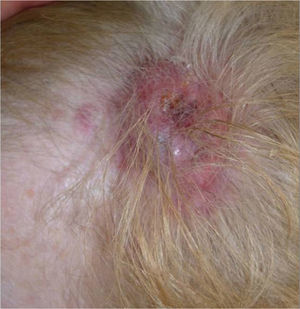
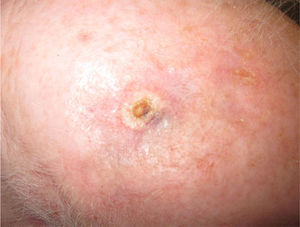
We present the case of a 68-year-old woman who consulted for an erythematous violaceous tumor with a diameter of 1cm on the scalp. Skin biopsy was consistent with a poorly differentiated angiosarcoma. The lesion grew rapidly and within a month it had progressed to a multilobulated, ulcerated tumor affecting the left side of the scalp and the right occipital region; also present in this region were red wine–colored macules measuring 1 to 2cm in diameter (Fig. 1).
A computed tomography (CT) scan ruled out distant disease and the patient was diagnosed with poorly differentiated angiosarcoma (T2b, N0, M0), classified as stage III according to the 2010 American Joint Committee on Cancer staging system.
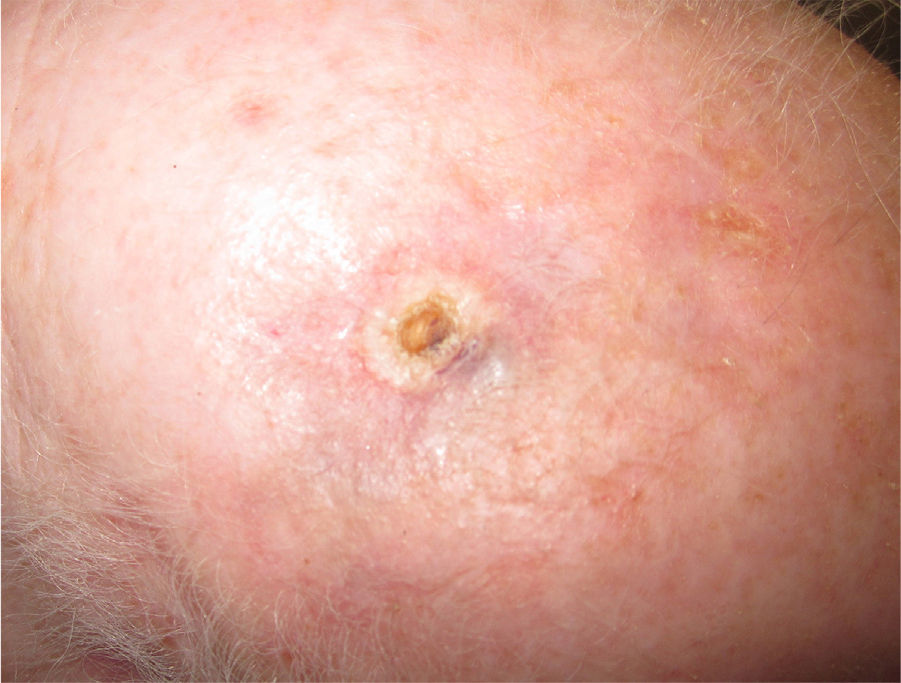
Immediate surgical treatment was ruled out due to the size of the lesion, which affected over half of the scalp. The patient was treated with neoadjuvant paclitaxel (80mg/m2/wk) and bevacizumab (10mg/kg every 2 weeks) and the lesion responded rapidly (Fig. 2). The treatment was completed after 14 cycles (each consisting of 1 bevacizumab infusion and 2 paclitaxel infusions) and was well tolerated. The remaining lesion was surgically removed, and no evidence of angiosarcoma was seen on histology. Despite the negative biopsy results for the area of the tumor, the patient was administered adjuvant radiation therapy (daily fractions of 2Gy, up to a total dose of 50Gy) combined with bevacizumab (5mg/kg every 2 weeks) due to the persistence of a red wine–colored macule in the left preauricular region. Complete response continued following completion of the 2 treatments.
Two months later, however, the patient's general health deteriorated and clinical recurrence of the angiosarcoma was noted in the left preauricular region. The CT scan showed bone metastasis. Combined treatment with paclitaxel and bevacizumab was reinitiated, but the disease progressed and the patient died 14 months after diagnosis.
Angiosarcoma is a rare but very aggressive tumor with high metastatic potential. There are 3 forms, but angiosarcoma of the head and neck–the classic form–is the most common.3 The treatment of choice is surgical excision with wide margins followed by adjuvant high-dose wide-field radiotherapy (>50Gy).1,4,5 Chemotherapy, however, is the only option for patients with advanced or unresectable angiosarcoma, but the effectiveness of different regimens has not been demonstrated in clinical trials.1,2 Additionally, chemotherapy is often limited because of its toxicity.1 The most widely used regimens are anthracyclines and taxanes, but response is limited. Prospective studies of paclitaxel in the treatment of cutaneous angiosarcoma have shown a mean survival time of 8 months and a mean disease-free period of 4 months.2
The need for new drugs with improved effectiveness and tolerance is thus clear. Interest is currently focused on angiogenesis inhibitors.
Angiosarcoma originates from vascular endothelial cells, and several factors have been implicated in its pathogenesis. One of the most important of these is VEGF-A,1,2 which is a growth factor involved in the development of new blood vessels through the stimulation of receptors with tyrosine-kinase activity on the surface of endothelial cells. VEGF-A overexpression by neoplastic cells, which has been demonstrated in angiosarcoma and other tumors (of the lung, breast, and colon/rectum) promotes angiogenesis and consequently leads to increased blood supply and faster tumor growth.2,6 Bevacizumab is a monoclonal antibody that targets VEGF-A, preventing it from binding to its receptor, and thereby inhibiting angiogenesis. Nonetheless, the potential for recurrence and the short disease-free periods observed suggest that there are other factors at play and that VEGF inhibition alone is not sufficient to stop the tumor from growing.7
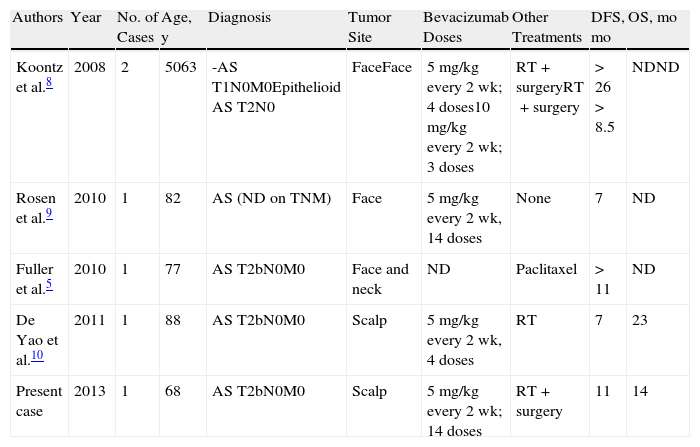
Five cases of cutaneous angiosarcoma treated with bevacizumab, alone or in combination, have been reported to date. A favorable response was reported in all cases, with good tolerance and longer survival than that seen with other treatments (Table 1).
Cases of Angiosarcoma (AS) Treated With Bevacizumab in the Literature.
| Authors | Year | No. of Cases | Age, y | Diagnosis | Tumor Site | Bevacizumab Doses | Other Treatments | DFS, mo | OS, mo |
| Koontz et al.8 | 2008 | 2 | 5063 | -AS T1N0M0Epithelioid AS T2N0 | FaceFace | 5mg/kg every 2 wk; 4 doses10mg/kg every 2 wk; 3 doses | RT+surgeryRT+surgery | >26>8.5 | NDND |
| Rosen et al.9 | 2010 | 1 | 82 | AS (ND on TNM) | Face | 5mg/kg every 2 wk, 14 doses | None | 7 | ND |
| Fuller et al.5 | 2010 | 1 | 77 | AS T2bN0M0 | Face and neck | ND | Paclitaxel | >11 | ND |
| De Yao et al.10 | 2011 | 1 | 88 | AS T2bN0M0 | Scalp | 5mg/kg every 2 wk, 4 doses | RT | 7 | 23 |
| Present case | 2013 | 1 | 68 | AS T2bN0M0 | Scalp | 5mg/kg every 2 wk; 14 doses | RT+surgery | 11 | 14 |
Abbreviations: AS, angiosarcoma; ND, no data; DFS, disease-free survival; OS, overall survival.
The results of the first phase II clinical trial on the use of bevacizumab alone to treat metastatic or locally advanced angiosarcoma were recently published by Agulnik et al.2 The results are promising, as half of the patients showed stable disease with a mean time to disease progression of 26 weeks.
Considering the scarcity of effective treatments and the results reported to date for bevacizumab in isolated cases and clinical trials, we believe that this drug could be considered in cases similar to the one we have presented. There are also clear indications that bevacizumab used in combination with other drugs could improve the prognosis in patients with angiosarcoma.
Please cite this article as: Nespereira-Jato MV, Peña-Panabad C, Quindós-Varela M, García-Silva J. Angiosarcoma irresecable tratado con bevacizumab y paclitaxel. Actas Dermosifiliogr. 2014;105:526–528.