Nail involvement is common in psoriasis and has a considerable impact on patient quality of life. Its clinical presentation depends on which part of the nail is affected: the bed or the matrix. Fifty percent of patients report associated pain. In this study, we analyzed the safety and effectiveness of tazarotene 0.1% in a hydrophilic ointment in the treatment of nail psoriasis.
Material and methodsWe performed an open observational study of 6 patients diagnosed with nail psoriasis. The patients applied a compounded preparation of tazarotene 0.1% ointment under occlusion every night for 6 months in their homes. They were not receiving any other topical or systemic treatments. Nail psoriasis severity (assessed using the Nail Psoriasis Severity Index [NAPSI]), subungual hyperkeratosis, onycholysis, splinter hemorrhages, oil stains, and nail pitting were evaluated at baseline and at 3 and 6 months.
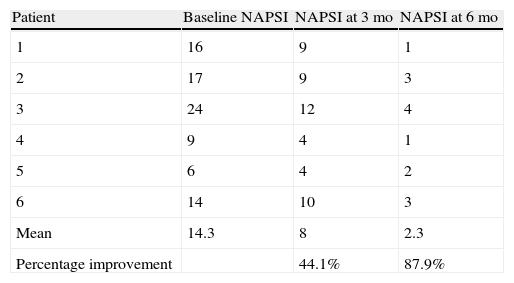
ResultsA statistically significant improvement between baseline and 6 months was observed in all patients: the mean (SD) NAPSI went from 14.3 (6.3; 95% CI, 11.74-16.92) to 2.3 (1.21; 95% CI, 1.84-2.3) while the median went from 15 to 2.5 (P = .007). The percentage improvement at the end of treatment was 87.9%. No adverse effects were observed.
ConclusionOur study shows the therapeutic potential of tazarotene ointment in nail psoriasis.
La afectación ungueal de la psoriasis es una presentación frecuente que interfiere de manera significativa en la calidad de vida de los pacientes. Su presentación clínica va a depender del área ungueal afecta: lecho o matriz. Un 50% de los pacientes refiere dolor asociado. En este estudio evaluamos la eficacia y seguridad del tazaroteno 0,1% en ungüento hidrófilo.
Material y métodosEstudio abierto y observacional de 6 pacientes diagnosticados de psoriasis ungueal. Se aplicó ungüento de tazaroteno 0,1% (fórmula magistral) en oclusión nocturna, en su domicilio durante 6 meses, sin otro tratamiento tópico o sistémico. Se determinó el Nail Psoriasis Severity Index (NAPSI) y se evaluaron la hiperqueratosis subungueal, onicólisis, hemorragias en astilla, manchas de aceite y piquiteado ungueal, en la visita basal, a los 3 y 6 meses.
ResultadosSe observó una mejoría estadísticamente significativa en todos los pacientes: NAPSI basal, media±DE 14,3± 6,3; IC 95% 11,74-16,92; mediana 15; NAPSI a los 6 meses: media±DE 2,3 ±1,21; IC 95% 1,84-2,83; mediana 2,5; p=0,007. El porcentaje de mejoría fue del 87,9% al final del tratamiento. No se registraron efectos adversos.
ConclusiónNuestro estudio muestra un potencial terapéutico del ungüento de tazaroteno en la psoriasis ungueal.
Nail psoriasis is a therapeutic challenge for the dermatologist; consequently, new approaches to this condition should be investigated. Psoriasis is one of the diseases that most affect the nail bed, and the nails are involved in 10% to 50% of patients with psoriasis; we have observed a frequency of 53% among our patients.1 The condition is less common in pediatric patients (7%-13%), but it increases to 80% in patients with psoriatic arthritis.1
Nail psoriasis has a considerable psychosocial impact that affects quality of life and alters the patient's body image; in addition, associated pain can lead to a reduction in activities of daily living.
The clinical manifestations of nail dystrophy range from the more common ones, such as pitting, onycholysis, and subungual hyperkeratosis, to the less common splinter hemorrhages in the nail bed.
Today, the use of new vehicles and active substances at higher concentrations leads to better transungual absorption and increased penetration, thus considerably improving adherence to treatment and efficacy.2 Tazarotene is a synthetic retinoid derived from vitamin A that has proven beneficial in modulating keratinocyte proliferation and in reducing inflammation, both of which are involved in the pathogenesis of nail psoriasis. It has beneficial effects on the matrix, bed, and periungual skin and induces few adverse effects.2
We analyze the efficacy and safety profile of tazarotene 0.1% hydrophilic ointment used to treat nail psoriasis.
Material and MethodsWe performed an open-label observational study of patients diagnosed with nail psoriasis in the dermatology clinic.
The study population comprised 6 patients with nail psoriasis affecting both the matrix and the bed. None of the patients was receiving systemic treatment or using topical agents.
Home treatment was started with tazarotene 0.1% ointment (compounded formula) applied under occlusive dressings at night for 6 months.
The study started in October 2009 and ended in April 2010. All the patients gave their consent to participate in the study.
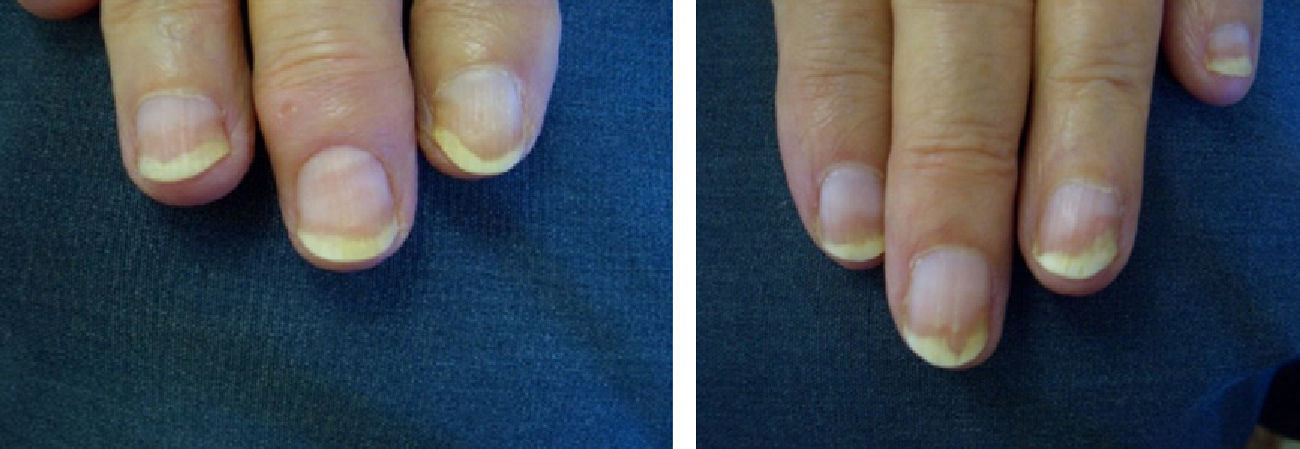
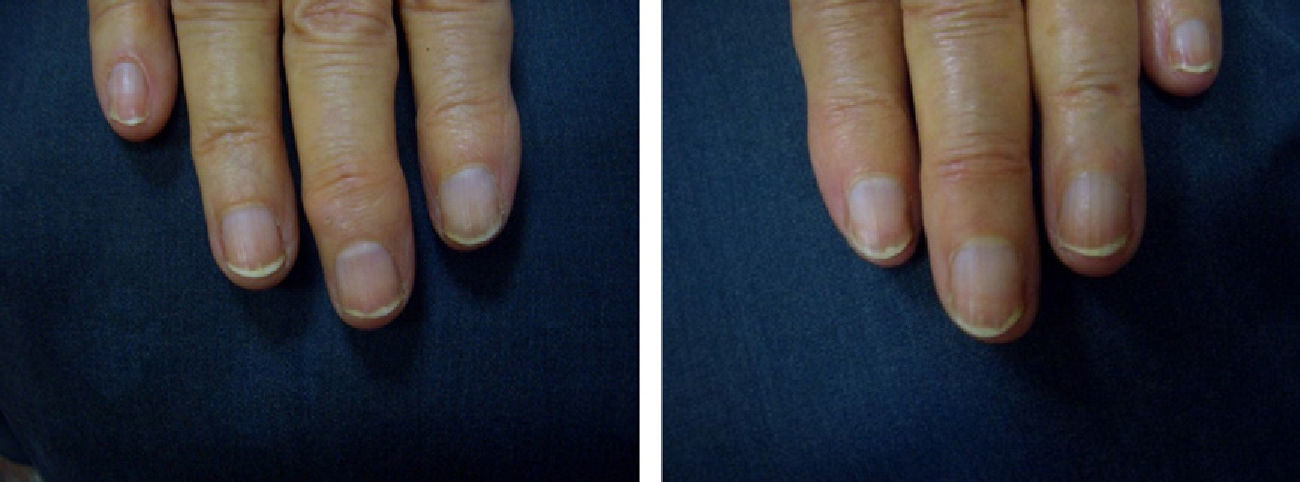
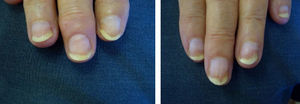
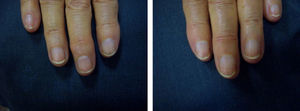
Patients underwent clinical assessment (including photographs of their lesions) at baseline, 3 months, and 6 months. The clinical parameters evaluated were subungual hyperkeratosis, onycholysis, splinter hemorrhage, oil spots, and pitting. The Nail Psoriasis Severity Index (NAPSI)3 was also calculated at baseline, 3 months, and 6 months by the same observer (MSR).
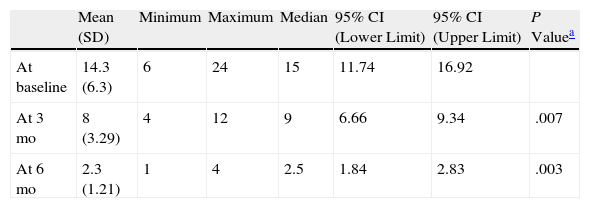
ResultsA statistically significant improvement in the lesions was observed at 3 months (P=.003); this improvement increased at 6 months (P=.007) (Figures 1 and 2, Table 1). The NAPSI fell from a mean (SD) of 14.3 (6.34) at baseline (median, 15.0; 95% CI, 11.74-16.92) to 2.33 (1.21) at 6 months (median, 2.5; 95% CI, 1.84-2.83) (P=.007). In percentage terms, the lesions had improved by 48% at 3 months and 88% at 6 months (Tables 1 and 2).
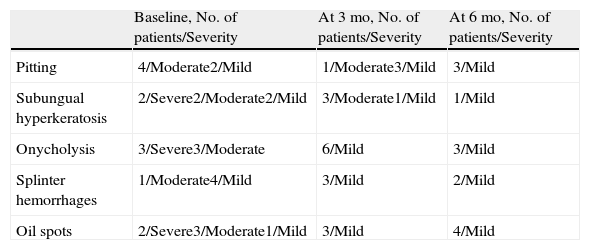
Nail Psoriasis Severity Index Over Time.
| Mean (SD) | Minimum | Maximum | Median | 95% CI (Lower Limit) | 95% CI (Upper Limit) | P Valuea | |
| At baseline | 14.3 (6.3) | 6 | 24 | 15 | 11.74 | 16.92 | |
| At 3 mo | 8 (3.29) | 4 | 12 | 9 | 6.66 | 9.34 | .007 |
| At 6 mo | 2.3 (1.21) | 1 | 4 | 2.5 | 1.84 | 2.83 | .003 |
Abbreviations: CI, confidence interval.
The most outstanding clinical feature in most cases was remission of subungual hyperkeratosis, onycholysis, and associated pain. Splinter hemorrhages disappeared in two-thirds of patients and oil spots in one-third. Pitting improved considerably in half of the patients (Table 3).
Change in the Severity of the Signs Over Time.
| Baseline, No. of patients/Severity | At 3 mo, No. of patients/Severity | At 6 mo, No. of patients/Severity | |
| Pitting | 4/Moderate2/Mild | 1/Moderate3/Mild | 3/Mild |
| Subungual hyperkeratosis | 2/Severe2/Moderate2/Mild | 3/Moderate1/Mild | 1/Mild |
| Onycholysis | 3/Severe3/Moderate | 6/Mild | 3/Mild |
| Splinter hemorrhages | 1/Moderate4/Mild | 3/Mild | 2/Mild |
| Oil spots | 2/Severe3/Moderate1/Mild | 3/Mild | 4/Mild |
No treatment-related adverse effects were observed in any patient.
DiscussionThe main topical treatments for nail psoriasis have traditionally comprised potent corticosteroids administered intralesionally and in the form of nail lacquer applied under an occlusive dressing.4,5
Corticosteroid infiltrations can cause considerable pain, atrophy, hypochromia, secondary infection, inclusion cysts, and tendon rupture. These agents are only recommended as a last resort in severe cases of intense trachyonychia, and only if 1 or 2 fingers are involved.
Favorable results have been observed with 8% clobetasol propionate lacquer, which improved pitting without producing atrophy.4 Compared with tazarotene 0.1% hydrophilic ointment, clobetasol has a more marked effect on psoriasis of the matrix, whereas tazarotene acts more on the nail bed.
Of the 3 vitamin D derivatives calcipotriol, tacalcitol, and calcitriol, the most widely studied in Spain is calcipotriol, which was effective in the nail bed (not in the matrix) and reduced hyperkeratosis. Calcipotriol should be administered in combination with corticosteroids.5–7
Psoralen (generally in the form of 8-methoxypsoralen) is another therapeutic option and can be administered topically or orally, followed by exposure to UV-A radiation (320-400nm). Before the advent of clobetasol lacquer and vitamin D derivatives, psoralen UV-A was widely used to treat severe nail psoriasis. This approach continues to be effective, especially when treatment is combined with acitretin. Psoralen UV-A is particularly effective if psoriasis presents with pustules and hyperkeratosis.8
Also noteworthy are the usefulness and the favorable outcome obtained with photodynamic therapy and pulsed dye laser, which have even proven effective for treating the matrix; however, administration of the former is uncomfortable, and the cost of the latter is high.9
The therapeutic potential of tazarotene 0.1% gel was well established in 2 studies showing its safety and tolerability.10,11 Other studies show the efficacy of tazarotene 0.1% cream on comparison with clobetasol 0.05% cream.2
Notwithstanding, to our knowledge, this is the first study to examine tazarotene 0.1% hydrophilic ointment.
We observed a clinically significant improvement in nail involvement, especially subungual hyperkeratosis and onycholysis, in all 6 patients.
Despite its small sample size, our study reveals that tazarotene 0.1% ointment is a well-tolerated option for the treatment of nail psoriasis.
More clinical studies with larger sample sizes should be performed to obtain more significant results.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fischer-Levancini C, Sánchez-Regaña M, Llambí F, Collgros H, Expósito-Serrano V, Umbert-Millet P. Psoriasis ungueal: tratamiento con ungüento. Actas Dermosifiliogr.2012;103:725-728.