The therapy of patients with psoriasis and liver disease can be a challenge due to the increased risk of adverse effects from traditional systemic treatments; in addition, although the anti-tumor necrosis factor agents are considered safer, they have also been associated with drug-induced liver injury and reactivation of viral hepatitis. Ustekinumab has a different mechanism of action and the little that is known of its effects on the liver comes from pivotal studies. The objectives of this study were to estimate the incidence of drug-induced liver injury in patients treated with ustekinumab in daily clinical practice and to analyze liver alterations in those patients with pre-existing liver disease.
MethodAll patients treated with the standard regimen of ustekinumab were included in the study. Variables gathered included age, sex, type of psoriasis, nail involvement, arthritis, previous treatments, history of liver disease, viral serology, Psoriasis Area Severity Index (at baseline and at 12, 16, and 52 weeks), transaminase levels, manifestations of liver disease, liver ultrasound, and factors such as body mass index, alcohol consumption, and ferritin levels.
ResultsGrade 1 elevation of the transaminases was only observed in 6 patients; no cases of severe hypertransaminasemia were observed. None of the patients with elevation of the transaminases at baseline developed problems during treatment.
ConclusionsUstekinumab-related liver injury is uncommon and mild. From a hepatic point of view, the drug appears safe, even in patients with pre-existing liver disease and those who have developed altered liver function previously with other drugs.
Los pacientes con psoriasis y hepatopatías son un reto terapéutico dado el mayor riesgo de efectos adversos que presentan con el tratamiento sistémico clásico y a que, aunque los fármacos anti-TNF-α son considerados más seguros, también se han descrito reactivaciones de hepatitis vírica y hepatitis tóxica en relación con los mismos. El ustekinumab (UTK) tiene un mecanismo de acción diferente y se sabe poco de sus efectos en el hígado, sobre todo a partir de los estudios pivotales. Nuestro objetivo ha sido estimar la incidencia de toxicidad hepática en los pacientes tratados con UTK en la práctica clínica habitual, y examinar los cambios hepáticos en aquellos pacientes tratados que tenían hepatopatía de base.
MétodoSe incluyeron todos los pacientes tratados con UTK según pauta habitual. Se analizaron la edad, el género, el tipo de psoriasis, la afectación ungueal, la artritis, los tratamientos previos, la hepatopatía previa, las serologías virales y el PASI (basal y a las 12, 16 y 52 semanas). Se analizaron también las transaminasas, los signos y síntomas de hepatopatía y los factores como el índice de masa corporal, el hábito enólico, la ferritina y la ecografía hepática.
ResultadosSe observaron hipertransaminasemias de grado 1 en solo 6 pacientes. Ninguno presentó hipertransaminasemias graves. Ninguno de los pacientes con hipertransaminasemia basal tuvo problemas durante el tratamiento.
ConclusionesLa hepatotoxicidad asociada a UTK es poco frecuente y grave, y parece seguro a nivel hepático, incluso en pacientes con hepatopatía basal o que habían presentado con anterioridad alteraciones hepáticas con otros fármacos.
The greater frequency of adverse effects with classic systemic treatments limits available therapeutic options in patients with psoriasis and liver disease.1 In the case of viral hepatitis, some drugs (interferon and ribavirin) can trigger or worsen pre-existing psoriasis,2 and immunosuppressive and hepatotoxic drugs are relatively contraindicated.2 Anti–tumor necrosis factor alfa (anti-TNF-α) agents are considered a safer option.3 Anti-TNF-α agents, especially etanercept, have generally proven successful in patients with hepatitis C virus infection.2,4,5 In contrast, viral replication has been reported in patients with hepatitis B virus infection and psoriasis who are taking anti-TNF-α agents,3 even in cases where the infection has resolved.6–8 Therefore, even though these patients are HBsAg-negative, they should be closely monitored.9 Furthermore, anti-TNF-α agents have exceptionally been associated with severe liver damage (liver failure, cholestasis, and autoimmune hepatitis).
Little is known about the effects on the liver of ustekinumab (UTK), an anti-p40 agent that has proven efficacious in patients with psoriasis. Normal transaminase values were recorded in the PHOENIX 1 study.10 In the PHOENIX 2 study, a patient from the placebo group developed hepatocellular carcinoma, and no significant differences were found between patients treated with placebo and patients treated with UTK.11 Similarly, in the ACCEPT study,12 no differences in transaminase values were recorded between UTK and etanercept. With respect to clinical practice, several recent series of patients treated with UTK did not reveal adverse liver effects,13–15 and only 1 study found elevated transaminases (44% of patients), although neither the levels nor the duration were specified.16
The primary objective of our study was to estimate the frequency of liver toxicity in patients with psoriasis treated with UTK according to habitual clinical practice; the secondary objective was to assess the affect of UTK on liver involvement in patients with liver disease or elevated transaminases before initiation of treatment.
Material and MethodsWe performed an observational, retrospective study of consecutive patients with moderate to severe psoriasis who started treatment with UTK between March 1, 2009 and June 30, 2011 at the Psoriasis Unit of Hospital de la Princesa, Madrid, Spain. The study was approved by the Ethics Committee of the Biomedical Research Foundation of Hospital la Princesa. Patients aged <18 years or whose clinical history lacked valid data were excluded. Clinical histories and additional test results were reviewed. The data recorded included height, weight, type of psoriasis, age at diagnosis, disease duration, nail involvement, psoriatic arthritis, Psoriasis Areas and Severity Index (PASI), body surface area (BSA), Dermatology Life Quality Index (DLQI), alcohol consumption, smoking, previous liver disease, previous biologic and systemic drugs, and concomitant medication (Table 1). The analytical data reviewed included a complete blood count, biochemistry with liver profile (aspartate aminotransferase [AST, reference range, 7-35U/L; alanine aminotransferase [ALT], reference range, 8-40U/L; and gamma glutamyl transpeptidase [GGT], reference range, 6-50U/L), lipid profile, serology (hepatitis B and C virus, human immunodeficiency virus [HIV]), result of a Mantoux test with booster, and chest radiograph. All patients received subcutaneous UTK (45mg) at weeks 0, 4, and every 12 weeks thereafter. Liver function was determined in all patients at 4 and 16 weeks and at most follow-up visits. Ultrasound examination of the liver was performed in patients with a previous history of liver disease or elevated transaminases and, during follow-up, in all those patients who presented altered transaminase levels or signs or symptoms suggestive of or compatible with liver disease.
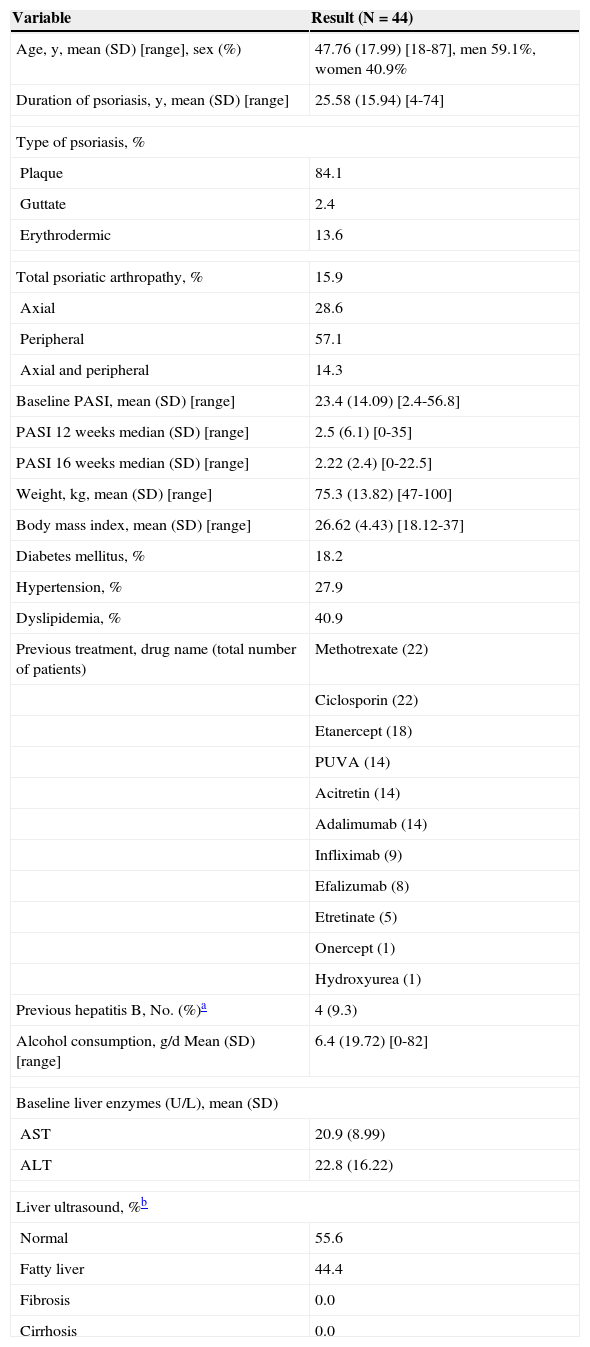
Baseline Data of a Cohort of 44 Patients Treated With Ustekinumab.
| Variable | Result (N=44) |
|---|---|
| Age, y, mean (SD) [range], sex (%) | 47.76 (17.99) [18-87], men 59.1%, women 40.9% |
| Duration of psoriasis, y, mean (SD) [range] | 25.58 (15.94) [4-74] |
| Type of psoriasis, % | |
| Plaque | 84.1 |
| Guttate | 2.4 |
| Erythrodermic | 13.6 |
| Total psoriatic arthropathy, % | 15.9 |
| Axial | 28.6 |
| Peripheral | 57.1 |
| Axial and peripheral | 14.3 |
| Baseline PASI, mean (SD) [range] | 23.4 (14.09) [2.4-56.8] |
| PASI 12 weeks median (SD) [range] | 2.5 (6.1) [0-35] |
| PASI 16 weeks median (SD) [range] | 2.22 (2.4) [0-22.5] |
| Weight, kg, mean (SD) [range] | 75.3 (13.82) [47-100] |
| Body mass index, mean (SD) [range] | 26.62 (4.43) [18.12-37] |
| Diabetes mellitus, % | 18.2 |
| Hypertension, % | 27.9 |
| Dyslipidemia, % | 40.9 |
| Previous treatment, drug name (total number of patients) | Methotrexate (22) |
| Ciclosporin (22) | |
| Etanercept (18) | |
| PUVA (14) | |
| Acitretin (14) | |
| Adalimumab (14) | |
| Infliximab (9) | |
| Efalizumab (8) | |
| Etretinate (5) | |
| Onercept (1) | |
| Hydroxyurea (1) | |
| Previous hepatitis B, No. (%)a | 4 (9.3) |
| Alcohol consumption, g/d Mean (SD) [range] | 6.4 (19.72) [0-82] |
| Baseline liver enzymes (U/L), mean (SD) | |
| AST | 20.9 (8.99) |
| ALT | 22.8 (16.22) |
| Liver ultrasound, %b | |
| Normal | 55.6 |
| Fatty liver | 44.4 |
| Fibrosis | 0.0 |
| Cirrhosis | 0.0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; PASI, Psoriasis Area Severity Index; PUVA, psoralen-UV-A.
Patients with a positive Mantoux result were treated with isoniazid (300mg/d) for 9 months; prophylaxis was started 1 month before initiation of UTK.
Elevated transaminases were classified according to severity as follows: grade 1 (up to 3 times the upper reference value); grade 2 (3-5 times the upper reference value); and grade 3 (5-10 times the upper reference value and acute hepatitis).17 Ultrasound examination of the liver enabled patients to be classed as healthy or ill (fatty liver disease, fibrosis, or cirrhosis).
The statistical analysis was performed using SPSS version 19 for Windows (SPSS Inc). Quantitative variables were analyzed using the t test. Binomial variables were analyzed using the chi-square test and a Fisher exact test when necessary.
ResultsThe study population comprised 44 patients, of whom 59% were men and the majority nonsmokers (57.5%). The mean follow-up time with UTK was 46.2 months (range, 25.3-63.4).
Most patients treated with UTK had plaque psoriasis; nail involvement was recorded in 58.8%. Table 1 shows baseline population data, previous biologic and systemic drugs used to treat psoriasis, and the percentage of patients with relevant comorbid conditions. Three patients had received a cumulative dose of methotrexate greater than 2g, although none had reached 3g. The mean cumulative dose was 190mg. Some patients simultaneously received hepatotoxic drugs such as statins (5 patients), methotrexate (2 patients), hypoglycemic agents (2 patients), and angiotensin II antagonists (1 patient).
Confounding factors for liver toxicity were analyzed. Only 4 patients had previously had hepatitis B infection; no patients had hepatitis C infection, HIV infection, or active hepatitis B infection. Mean ferritin was 108.92mg/L (range, 15-366mg/L) in the 24 patients studied (local reference value, 15-150mg/mL). Only 1 patient had elevated ferritin (366mg/L), although hemochromatosis was ruled out. Two patients were obese (body mass index [BMI] >30). Ultrasound examination of the liver revealed fatty liver in 9 patients. No patients had fibrosis or cirrhosis at baseline.
As for previous liver disease, 41.9% of patients had had elevated transaminases associated with other drugs such as infliximab (5 cases), etanercept (3 cases), adalimumab (2 cases), and isoniazid (2 cases).
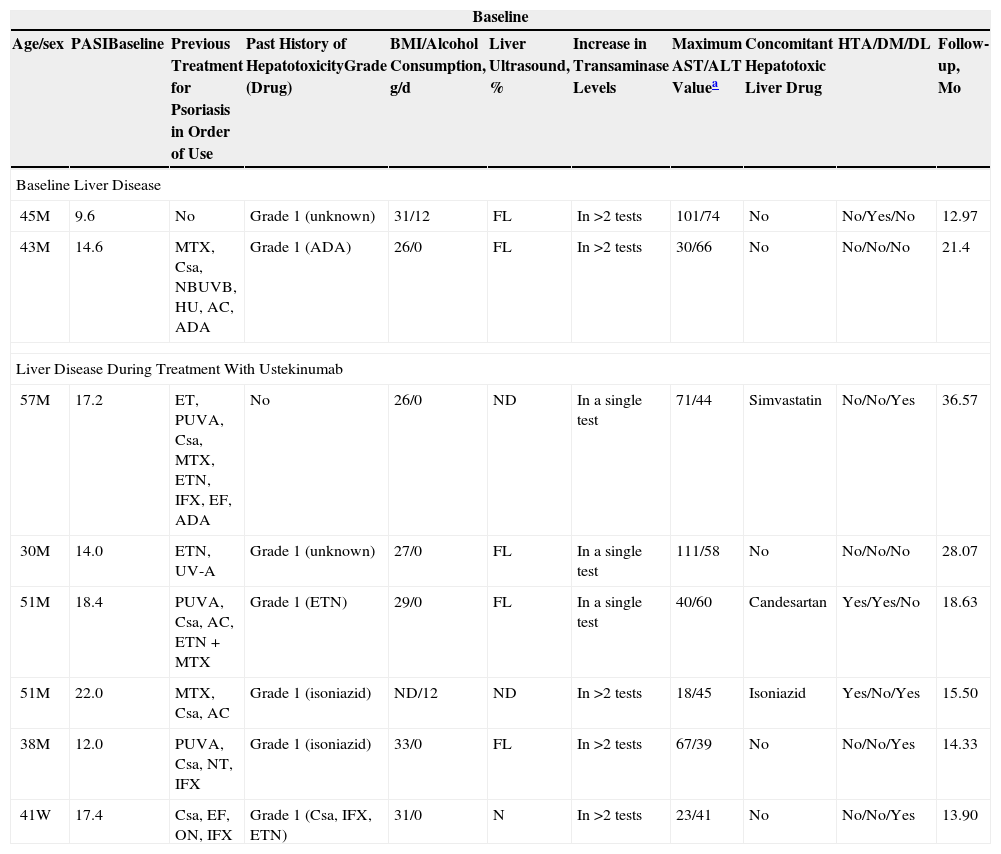
Two patients started UTK with increased baseline transaminases (Table 2). The first was a 45-year-old man with a 15-year history of moderate to severe psoriasis and a clinical history that was unremarkable except for chronic alcoholism and obesity (BMI, 31.5). When the patient started UTK, the values for severity of psoriasis were as follows: baseline PASI, 9.6; BSA, 7.1%; and DLQI, 13. At 16 weeks of treatment these values were as follows: PASI, 3; BSA, 3%; and DLQI, 1. Levels of AST and ALT remained stable during treatment, with a slight upward trend. The second patient was a 43-year-old man with fatty liver disease and a BMI of 26.2. He did not consume alcohol, had elevated transaminases, and had been treated with ciclosporin, retinoids, narrowband UV-B therapy, and methotrexate. His transaminase levels had fallen gradually but returned to normal after 19 months of treatment with UTK.
Relevant Demographic Data and Data on Previous Liver Toxicity, BMI, and Other Comorbidities. Maximum Transaminase Values in Patients Treated With Ustekinumab Who Presented Liver Disease at Baseline or Who Developed Liver Disease During Treatment.
| Baseline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age/sex | PASIBaseline | Previous Treatment for Psoriasis in Order of Use | Past History of HepatotoxicityGrade (Drug) | BMI/Alcohol Consumption, g/d | Liver Ultrasound, % | Increase in Transaminase Levels | Maximum AST/ALT Valuea | Concomitant Hepatotoxic Liver Drug | HTA/DM/DL | Follow-up, Mo |
| Baseline Liver Disease | ||||||||||
| 45M | 9.6 | No | Grade 1 (unknown) | 31/12 | FL | In >2 tests | 101/74 | No | No/Yes/No | 12.97 |
| 43M | 14.6 | MTX, Csa, NBUVB, HU, AC, ADA | Grade 1 (ADA) | 26/0 | FL | In >2 tests | 30/66 | No | No/No/No | 21.4 |
| Liver Disease During Treatment With Ustekinumab | ||||||||||
| 57M | 17.2 | ET, PUVA, Csa, MTX, ETN, IFX, EF, ADA | No | 26/0 | ND | In a single test | 71/44 | Simvastatin | No/No/Yes | 36.57 |
| 30M | 14.0 | ETN, UV-A | Grade 1 (unknown) | 27/0 | FL | In a single test | 111/58 | No | No/No/No | 28.07 |
| 51M | 18.4 | PUVA, Csa, AC, ETN+MTX | Grade 1 (ETN) | 29/0 | FL | In a single test | 40/60 | Candesartan | Yes/Yes/No | 18.63 |
| 51M | 22.0 | MTX, Csa, AC | Grade 1 (isoniazid) | ND/12 | ND | In >2 tests | 18/45 | Isoniazid | Yes/No/Yes | 15.50 |
| 38M | 12.0 | PUVA, Csa, NT, IFX | Grade 1 (isoniazid) | 33/0 | FL | In >2 tests | 67/39 | No | No/No/Yes | 14.33 |
| 41W | 17.4 | Csa, EF, ON, IFX | Grade 1 (Csa, IFX, ETN) | 31/0 | N | In >2 tests | 23/41 | No | No/No/Yes | 13.90 |
Abbreviations: AC, acitretin; ADA, adalimumab; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; Csa, ciclosporin; DM, diabetes mellitus; EF, efalizumab; ET, etretinate; ETN, etanercept; FL, fatty liver; HT, hypertension; HU, hydroxyurea; IFX, infliximab; MTX, methotrexate; N, normal, no abnormalities; NBUVB, narrowband UV-B therapy; ND, not done; ON, onercept; PUVA, psoralen-UV-A.
During treatment, 6 patients had grade 1 elevated transaminases (Table 2). Five had had liver toxicity associated with other drugs used to treat psoriasis, 3 had fatty liver disease, and 3 were receiving hepatotoxic agents concomitantly with UTK. The increase in transaminase levels occurred between 1 and 17 months after starting treatment (mean [SD], 3 [1.4]). Increased GGT was recorded in another patient. No patients showed signs or symptoms of liver disease or had to suspend or modify treatment because of liver toxicity. No severe adverse liver effects or cases of viral reactivation were detected during treatment with UTK.
The statistical analysis did not reveal statistically significant differences between patients with or without altered transaminase levels in terms of age (P=.592), alcohol consumption (P=.541), or BMI (P=.116). Similarly, no differences were detected for the percentage of patients who reached PASI 75 at 16 weeks (P=.597).
DiscussionGiven the mechanism of action of UTK,18,19 some liver effects are to be expected during treatment. In hepatitis B infection, the increase in interleukin (IL) 12 levels is correlated with hepatolysis, viral clearance,18 and sustained control of viremia in patients with chronic disease.19 The exact mechanism is unknown, because the activity of the IL-23/IL-17 proinflammatory pathway is also overexpressed in hepatitis B virus infection and could facilitate the chronic nature of the process.20 Type 17 helper T-cell (TH17) counts are increased in chronic hepatitis B virus infection, and the increase in the TH17/regulatory T-cell ratio can indicate poor prognosis.21 In hepatitis C virus infection, virus-induced activation of TH17 cells can contribute to viral clearance, because IL12p40 levels are higher in patients who respond to interferon alfa,22 although Zhang et al.23 did not confirm this observation. Polymorphisms in IL12p40 can modulate hepatitis C virus infection24 and account for the inconsistencies in published results. In the light of basic research data, UTK should be used with caution in patients with viral hepatitis, and although some series indicate that it is safe in clinical practice,25 reactivation of hepatitis B and C virus has been reported.26,27 We found no cases of reactivation of previous hepatitis B virus.
Increased IL17 levels have been detected in the liver of patients with nonalcoholic fatty liver disease and can facilitate the transition to steatohepatitis.28
IL-12 is also involved in other liver diseases and played a key role in the development of autoimmune cholangitis in a murine primary biliary cirrhosis model.29 Finally, TH17 cells are involved in rejection of liver allografts in rats.30 Therefore, modulation of TH17 could lead to improvement in many liver diseases. We did not observe any worsening of liver disease that was present at the start of treatment with UTK.
Comorbid conditions in patients with psoriasis have been widely studied.31,32 In our series, 42% of the patients were smokers, as reported elsewhere.33 Mean alcohol consumption was 6.36 g/d, which is lower than usually reported by other studies showing higher consumption in patients with psoriasis.34 In addition to being cytotoxic in the liver and in bone marrow, alcohol has an immunosuppressive effect. In fact, consuming more than 2-3 alcoholic drinks per week significantly increases the risk of psoriasis,34,35 and more than 100 g/d in men is a risk factor for increased activity of psoriasis.36 Other authors found no association between alcohol consumption and severity of psoriasis. The fact that we took a clinical history could have influenced the levels of alcohol consumption reported by the patients.
The prevalence of obesity has been reported to be double in patients with psoriasis, as has an association between BMI and PASI, namely, poorer disease control in patients with a high BMI.33 In our study, 20.5% of patients were obese (the prevalence of obesity in the general population in Spain was 13.3% during the same period37). These data agree with those reported by Kim et al.33
Nonalcoholic fatty liver disease is a chronic metabolic disease associated with both metabolic syndrome and with insulin resistance. It is the main cause of elevated transaminases in Europe.33 Of the 8 patients with nonalcoholic fatty liver disease in our study, only the eldest (62 years) had developed diabetes and dyslipidemia. Gisondi et al.38 reported that up to 50% of cases of nonalcoholic fatty liver disease are not diagnosed. This finding is consistent with ours and suggests that psoriasis could be a risk factor for onset, although other authors would require patients with high alcohol consumption to be ruled out.39 None of the patients with nonalcoholic fatty liver disease in our study consumed high amounts of alcohol, although 5 were obese. This finding is consistent with those of Gisondi et al.
Drug-induced liver toxicity is defined as a >3-fold increase in normal transaminase levels or twice the normal levels of bilirubin.40 We detected no cases of drug-induced liver toxicity. Elevated transaminases have been observed during treatment with anti-TNF-α agents,41 especially with infliximab,3 but also with etanercept and adalimumab.42 In our series, elevated transaminases were observed in 14.3% of patients during treatment with UTK. Despite reports of anti-TNF-α–induced liver toxicity in patients treated with a drug from the same family without recurrences,41,43–45 using drugs with a different mechanism of action seems safer.
Lastly, 5 of the patients in the present study were treated with statins, which induce liver toxicity and cause liver damage in 1% of cases. Grade 1 elevated transaminase levels were observed in a single analysis in only 1 patient, although this abnormality resolved without sequelae. Therefore, statins do not appear to be contraindicated in our series or in the literature reviewed.21
Our study is limited by sample size, short follow-up, and absence of data on the dose of previously prescribed hepatotoxic agents.
Our findings show that liver toxicity associated with UTK is mild and uncommon. Similarly, UTK seems to be a safe alternative in patients with previous liver disease or in those who have experienced liver alterations during treatment with antipsoriatic drugs, including anti-TNF-α agents.
Ethical DisclosuresProtection of Persons and AnimalsThe authors declare that this research did not involve experiments performed on humans or animals.
Confidentiality of DataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestM Llamas-Velasco has participated in clinical trials and advisory boards for AbbVie, Janssen-Cilag, and Novartis.
E Daudén has participated in advisory boards and clinical trials, acted as a consultant, and received grant support, research support, and fees for talks from the following companies: AbbVie Astellas, Biogen, Centocor Ortho Biotech Inc., Galderma, Glaxo, Janssen-Cilag, Leo Pharma, Merck-Serono, Novartis, Pfizer, Schering-Plough, Stiefel, Wyeth Pharmaceuticals, 3M, and Celgene.
A García-Diez has retired and has no conflicts of interest.
María José Concha-Garzón declares that she has no conflicts of interest.
Please cite this article as: Llamas-Velasco M, Concha-Garzón MJ, García-Diez A, Daudén E. Análisis de la hepatotoxicidad en psoriasis tratada con ustekinumab. Estudio retrospectivo de 44 pacientes en práctica clínica habitual. Actas Dermosifiliogr. 2015;106:470–476.