Dermatofibrosarcoma protuberans (DFSP) is a fibrohistiocytic tumor of intermediate malignancy that is very rare in childhood. Only 6% of these tumors present in children. Clinical diagnosis is very difficult in the early stages of disease, but to ensure appropriate treatment it is important to identify DFSP as early as possible and rule out benign conditions that are more common at this age.
The clinical presentation and histopathologic and molecular characteristics of DFSP are similar in children and adults. Clinical diagnosis is, however, more difficult in children and requires a high degree of suspicion. The absence of characteristic features and the rarity of this tumor explain why diagnosis is often delayed. Complete surgical excision of the tumor is very important to reduce the risk of recurrence.
This article presents a review of current knowledge about the management of DFSP in children and examines the latest treatment options.
El dermatofibrosarcoma protuberans es un tumor fibrohistiocitario de grado intermedio de malignidad, muy infrecuente en la infancia, con tan solo un 6% de estos tumores diagnosticados en la edad pediátrica. El diagnóstico clínico en los estadios iniciales es muy difícil, pero es necesario realizarlo lo más precozmente posible, así como excluir otros procesos benignos que son más frecuentes en la infancia para asegurar un tratamiento correcto.
Tanto la presentación clínica como la histopatología y las anomalías moleculares en los niños son similares a las que encontramos en los adultos. Sin embargo, el diagnóstico inicial es más difícil y requiere un alto índice de sospecha por parte del dermatólogo. La ausencia de rasgos característicos, junto a la rareza de este cuadro, conducen en muchas ocasiones a un retraso en el diagnóstico. Es muy importante realizar una extirpación quirúrgica completa del tumor para reducir el riesgo de recidiva.
Este artículo proporciona una revisión de los actuales conocimientos y opciones terapéuticas más novedosas en el manejo del dermatofibrosarcoma protuberans infantil.
Dermatofibrosarcoma protuberans (DFSP) is an intermediate-grade soft-tissue tumor, described by Darier and Ferrand1 in 1924. DFSP is characterized by a high incidence of local recurrences and a low metastatic potential.
This fairly rare tumor accounts for approximately 4% of all soft tissue sarcomas2 and has a prevalence of 0.1% among all malignant skin tumors in adults.3,4 The incidence in children is unknown, but it appears to be lower than in adults and a high degree of clinical suspicion is therefore required for diagnosis.5,6
The clinical presentation, the histopathologic and immunohistochemical characteristics, and molecular abnormalities in DFSP in childhood are similar to those observed in adults; likewise the same translocation between chromosomes 17 and 22 and their association with the product of the COL1A1-PDGFB fusion are observed.7,8 However, the site of the tumor is different in children, in whom it has a greater propensity to appear on the legs and acral regions.7
Clinical diagnosis in pediatric patients is much more difficult, and so there may be substantial delays in treatment. In this article, we will perform an extensive review of the literature on DFSP in this age group.
EpidemiologyIn children, the incidence of this tumor has been reported as higher among girls5,9 or the same in both sexes.7 It is also reported more frequently in blacks.4,10,11 In large series of patients, an incidence of 0.8 to 5 cases per year per million inhabitants has been reported.4,12,13
DFSP presents more frequently between 20 and 50 years of age, and presentation during childhood is rare: only 6% of tumors are found in patients under 16 years of age.4,14 However, a substantial proportion of DFSP lesions diagnosed in adults had begun to manifest in childhood or even at birth. The lack of clinical suspicion may therefore lead to long delays in diagnosis and so the prevalence of DFSP in childhood is probably underestimated.9,15 In a review published in 2010, Gooskens et al.6 analyzed reports of 166 cases in children. Of these, 38 were congenital cases.
PathogenesisThe histogenesis of DFSP is a subject of debate. DFSP is probably a slow-growing tumor originating in fibroblasts or histiocytes3,16 or arising from an undifferentiated mesenchymal cell with fibroblast, muscle-like, or neural-like characteristics.3,11Trauma has been considered a possible etiologic factor given that 16.5% of a series of 115 patients reported prior injury in the region of the lesion.17 Although trauma may be a coincidental finding that only reflects a prior lesion, reports of DFSP on surgical scars, old burns, and vaccination sites represent evidence in favor of a relationship between the two.18–20
In most children, there are specific cytogenetic abnormalities in the tumor cells, and translocations in chromosomes 17 and 22 in particular. This topic will be dealt with in more detail later.
In congenital cases, chromosomal abnormalities occur in utero, although the mechanism driving the change is not known. No epidemiological data have enabled identification of any predisposing or environmental risk factor for the development of DFSP during gestation.9
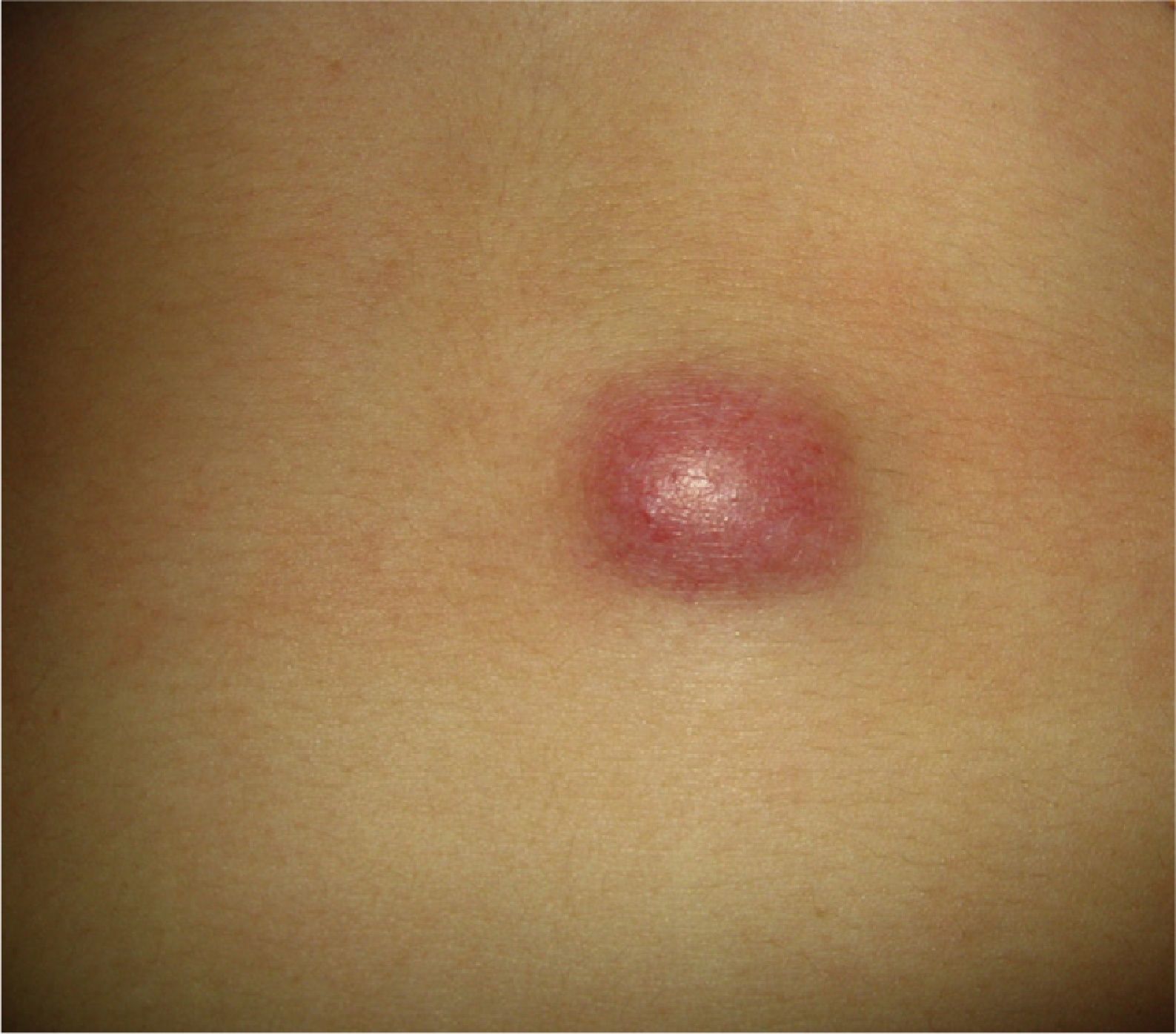
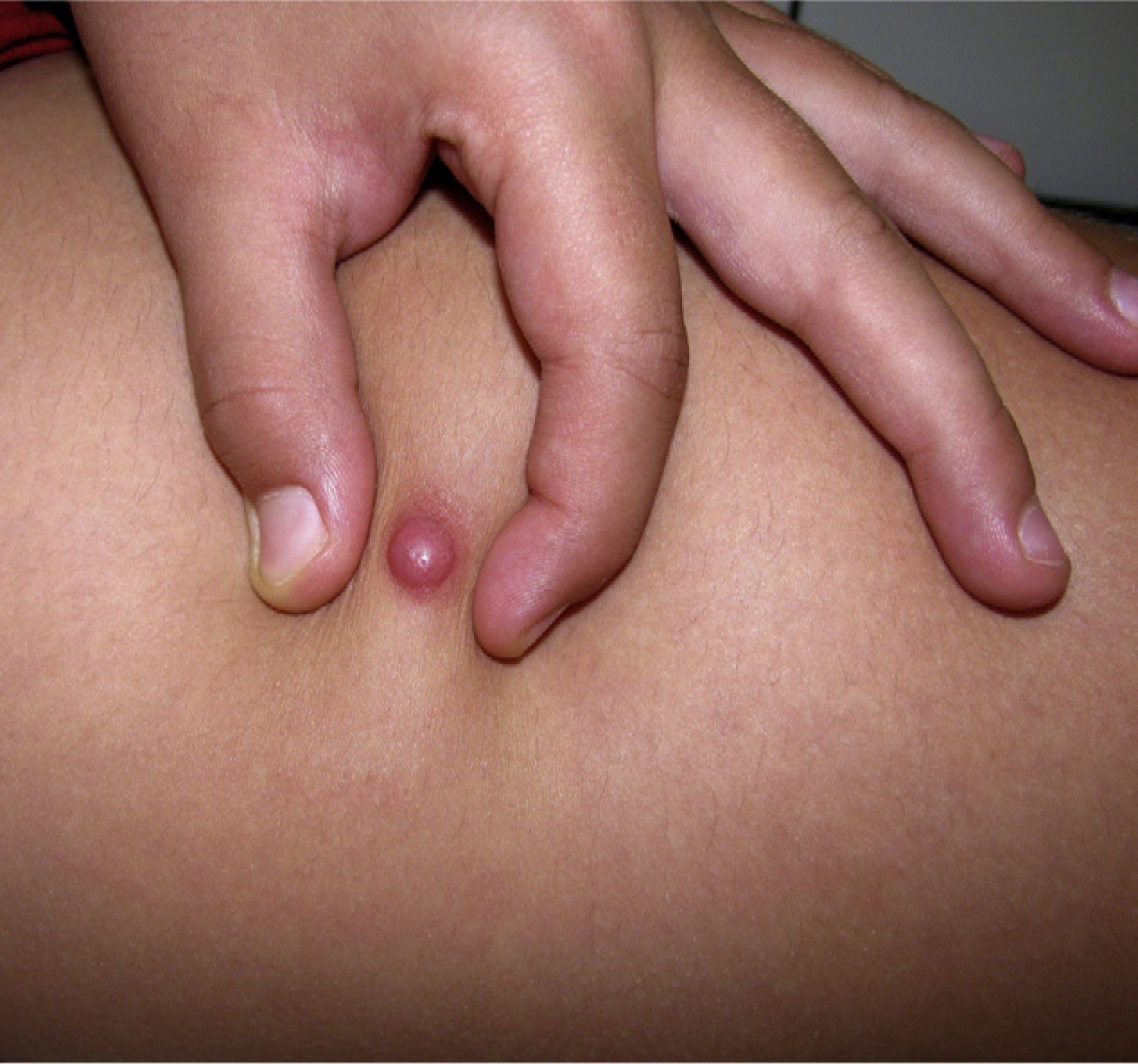
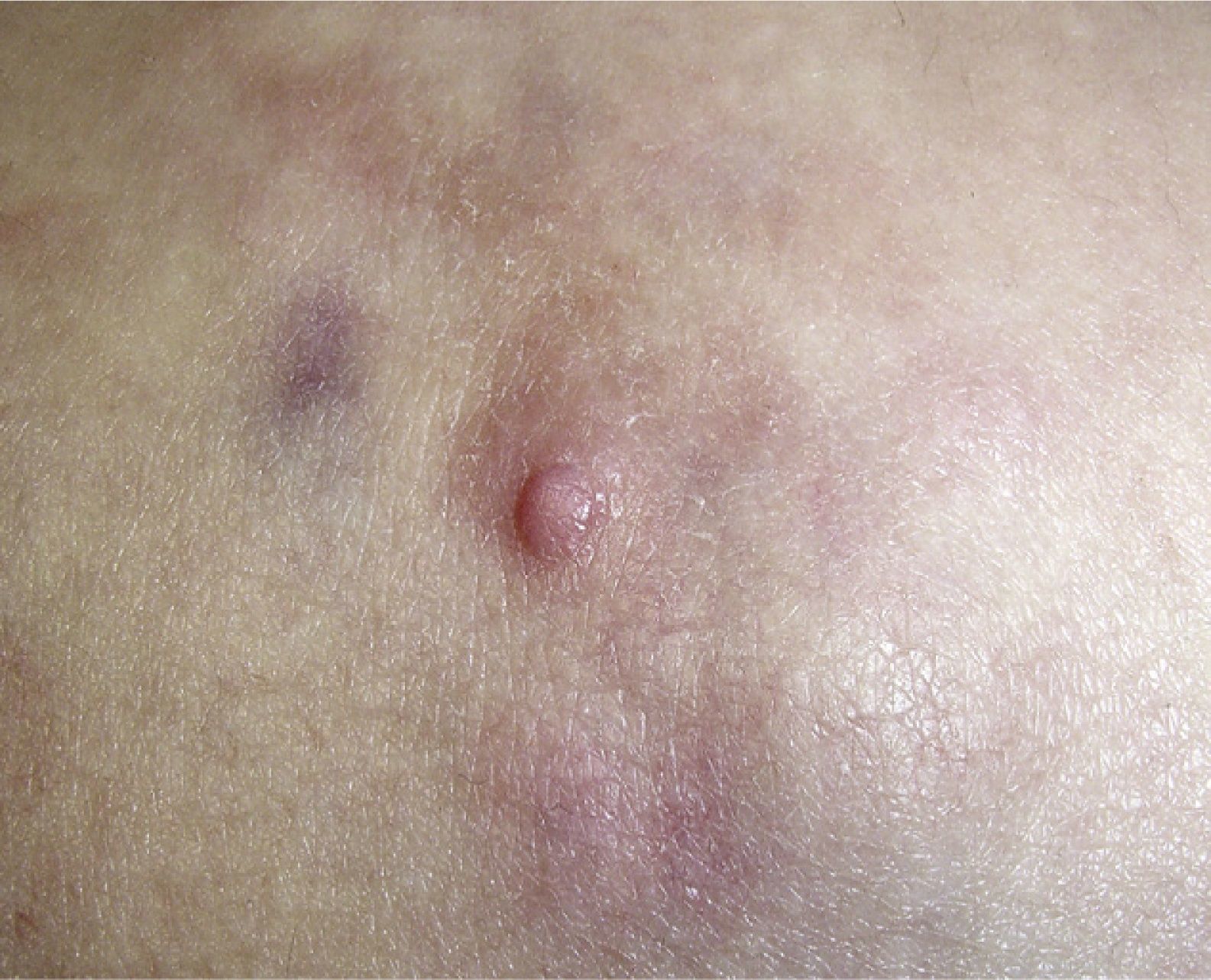
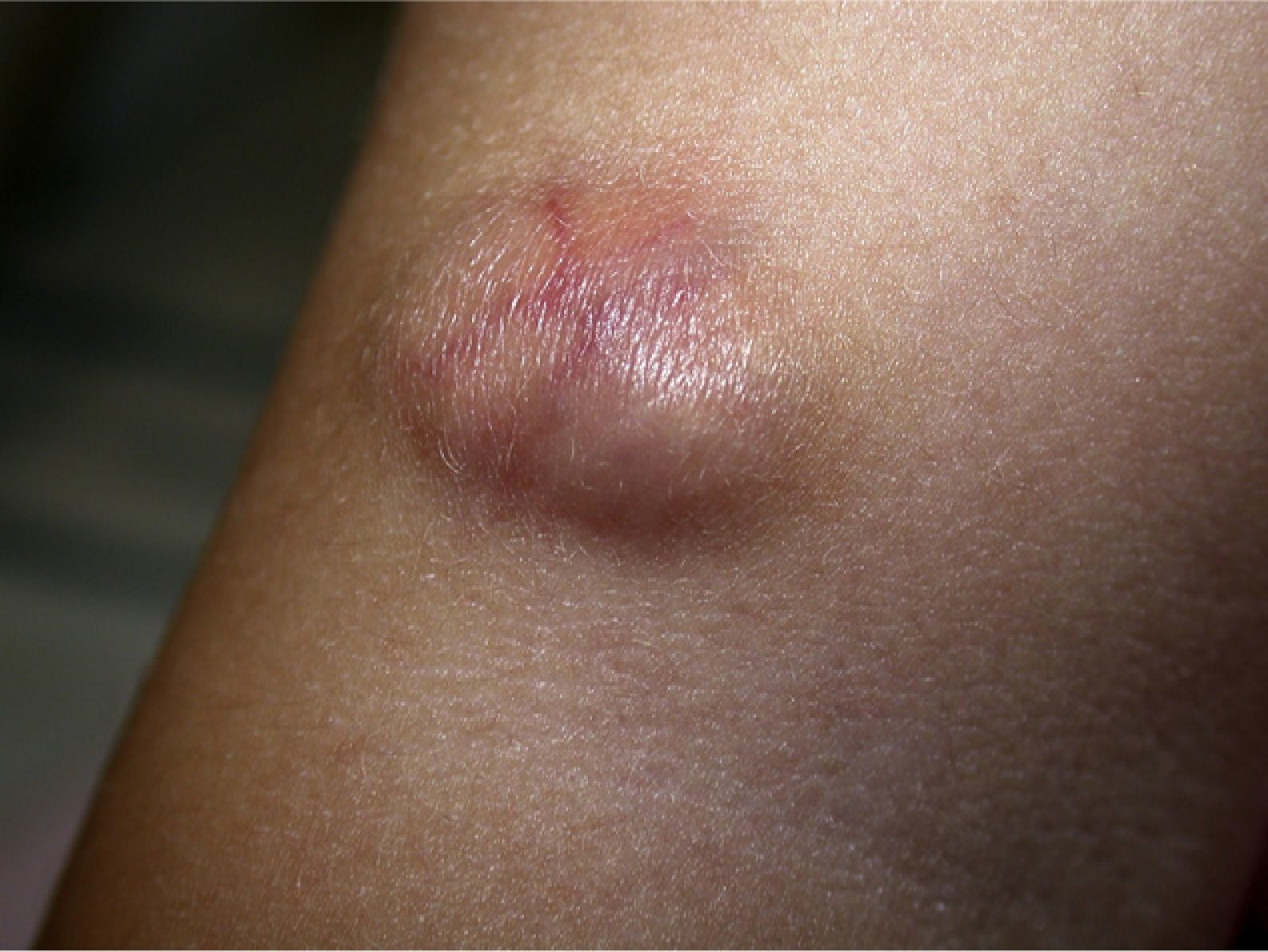
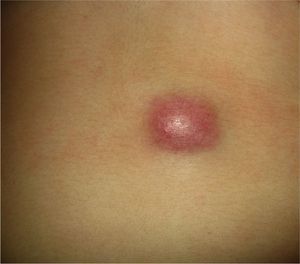
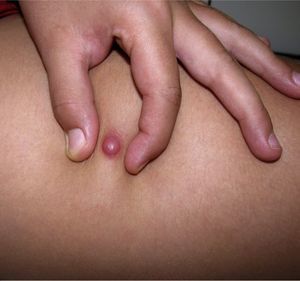
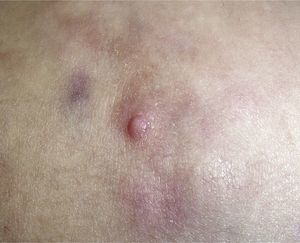
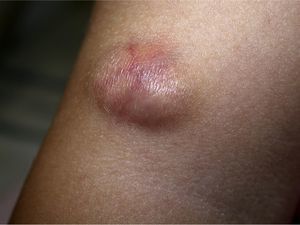
Clinical CharacteristicsThe clinical presentation during childhood is similar to the presentation in adults, and depends on the stage of growth. In the early stages, lesions in the form of single papules or plaques predominate, with deep-seated nodules being less common (Fig. 1).5 One of the most consistent features of this tumor is induration (Fig. 2). Normally the lesion moves freely over deep tissue structures until late-stage disease, when invasion of the underlying structures occurs.16 The overlying skin has an erythematous, brownish, violaceous, or flesh coloration. A bluish tinge may be the first manifestation of the tumor, and lead to an erroneous initial diagnosis of a vascular lesion (Fig. 3).15 Normally, lesions are asymptomatic and measure between 1 and 5cm on diagnosis. These lesions tend to grow progressively.21
Differential clinical diagnosis should include tumors and vascular malformations, keloids, scars, cystic hygroma, lipoid necrobiosis, infantile myofibroma, dermatofibroma, rhabdomyosarcoma, and pilomatricoma (Table 1).15
Differential Clinical Diagnosis of Childhood Dermatofibrosarcoma Protuberans.
| Macular, papular, nodular Lesions |
| • Vascular tumors |
| • Vascular malformations |
| • Keloids and scars |
| • Cystic hygroma |
| • Infantile myofibroma |
| • Dermatofibroma |
| • Rhabdomyosarcoma, pilomatrixoma |
| • Lipoid necrobiosis |
| Atrophic and anetodermic lesions |
| • Morphea |
| • Anetoderma |
| • Atrophoderma |
| • Lipoatrophia |
| • Scarring |
| • Lymphocytomas |
| • Morpheaform basal cell carcinoma |
| Congenital forms |
| • Vascular malformations |
| • Vascular tumors |
| • Infantile fibromatosis or myofibromatosis |
| • Fibrosarcoma |
| • Fibrous hamartoma |
| • Aplasia cutis |
| • Subcutaneous fat necrosis |
| • Arthropod bites |
| • Intrauterine trauma |
An atrophic variant is frequently encountered, particularly in congenital forms. In this variant, depressed plaques with a sclerotic appearance tend to remain flat. Also common among congenital tumors are anetodermic variants in the form of depressed plaques with a soft consistency.22,23 According to some authors, these variants represent early clinical forms of DFSP in childhood.24 Clinically, these lesions are confused with morpheic plaques, anetoderma, atrophoderma, lipoatrophia, scarring, lymphocytomas, atrophic dermatofibroma or, less often, morpheaform basal cell carcinoma.15,21,25,26
Regardless of the initial clinical presentation, after a variable period of up to 60 years, the lesion begins to grow more quickly, with the appearance on the surface of multiple protruding nodules, which give the name to the disease.15 At this stage, complications such as bleeding, ulceration, and pain may arise.10,15 Often, in these stages, the tumor invades deep structures such as the fascia, muscle, or bone.16
As in adults, most lesions present on the trunk and the proximal aspects of the limbs.3,15 The most common sites in children are the back3,26 and legs.7,11 Unlike adults, children commonly have tumors on acral areas, providing support for the suggestion that injury can trigger DFSP.21,24 In a review of 27 cases of DFPS in childhood, 14.8% were located on the hands and feet.27 In 2006, other authors reviewed all 150 cases in the literature and estimated this figure to be lower (9%).28
As mentioned above, tumors may be present from birth, and 38 to 61 such cases have been included in published series.6,13 Diagnostic delay in such cases—the mean time between appearance of the lesion and diagnosis is 14 years—is surprising, and is usually attributable to parental delay in consulting the dermatologist given the apparently harmless nature of the lesion. Delay has also been due to erroneous initial histologic diagnosis, in particular before the CD34 marker was used.9,13 Congenital DFSP lesions are also more frequently located on the trunk and proximal aspect of the limbs.9,26 The clinical appearance is very variable, with some lesions appearing as a erythematous-violaceous nodular plaque (the most common presentation), solitary tumors, dyschromic patches, and atrophic, sclerotic, or anetodermic plaques. It has been reported as associated with the rope necklace sign.26 DFSP is often mistaken for malformations or vascular tumors, infantile fibromatosis or myofibromatosis, fibrosarcoma or fibrous hamartoma, aplasia cutis, subcutaneous fat necrosis, arthropod bites, or intrauterine trauma.13,26 Radiologic studies can be useful in these patients, but the gold standard for diagnosis is skin biopsy and immunohistochemical and molecular studies.9,26
It is important to emphasize how difficult it is to diagnose this tumor in the nonprotuberans phase. Given how rare the tumor is in children, diagnosis may be delayed until adulthood, when the lesion has its characteristic bulging form and adopts its usual proliferative behavior.29 The mean time taken to arrive at a definitive diagnosis in some pediatric series is 5 years,13,29 though some children are not diagnosed until 14 or 15 years have elapsed.9,15
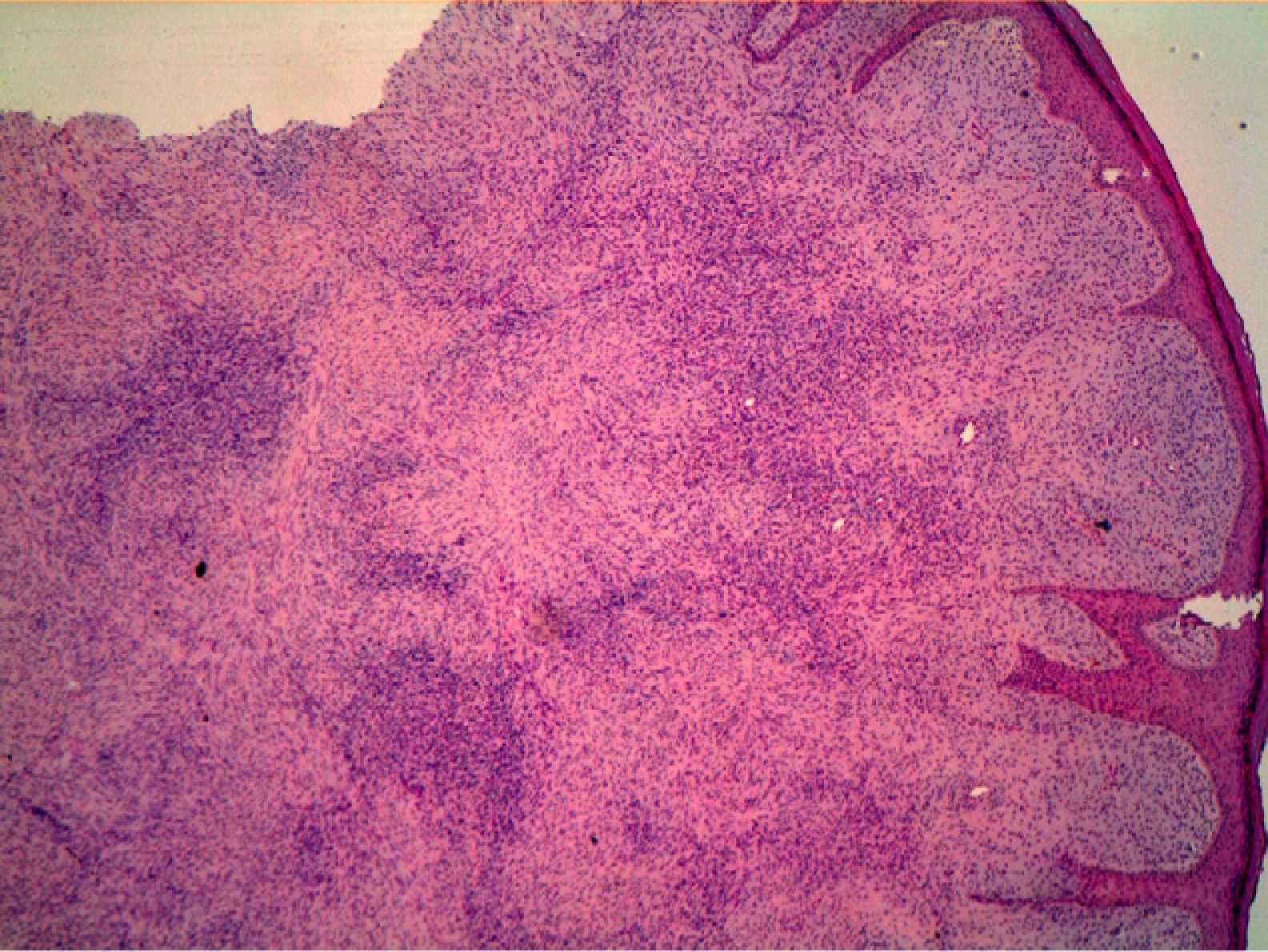
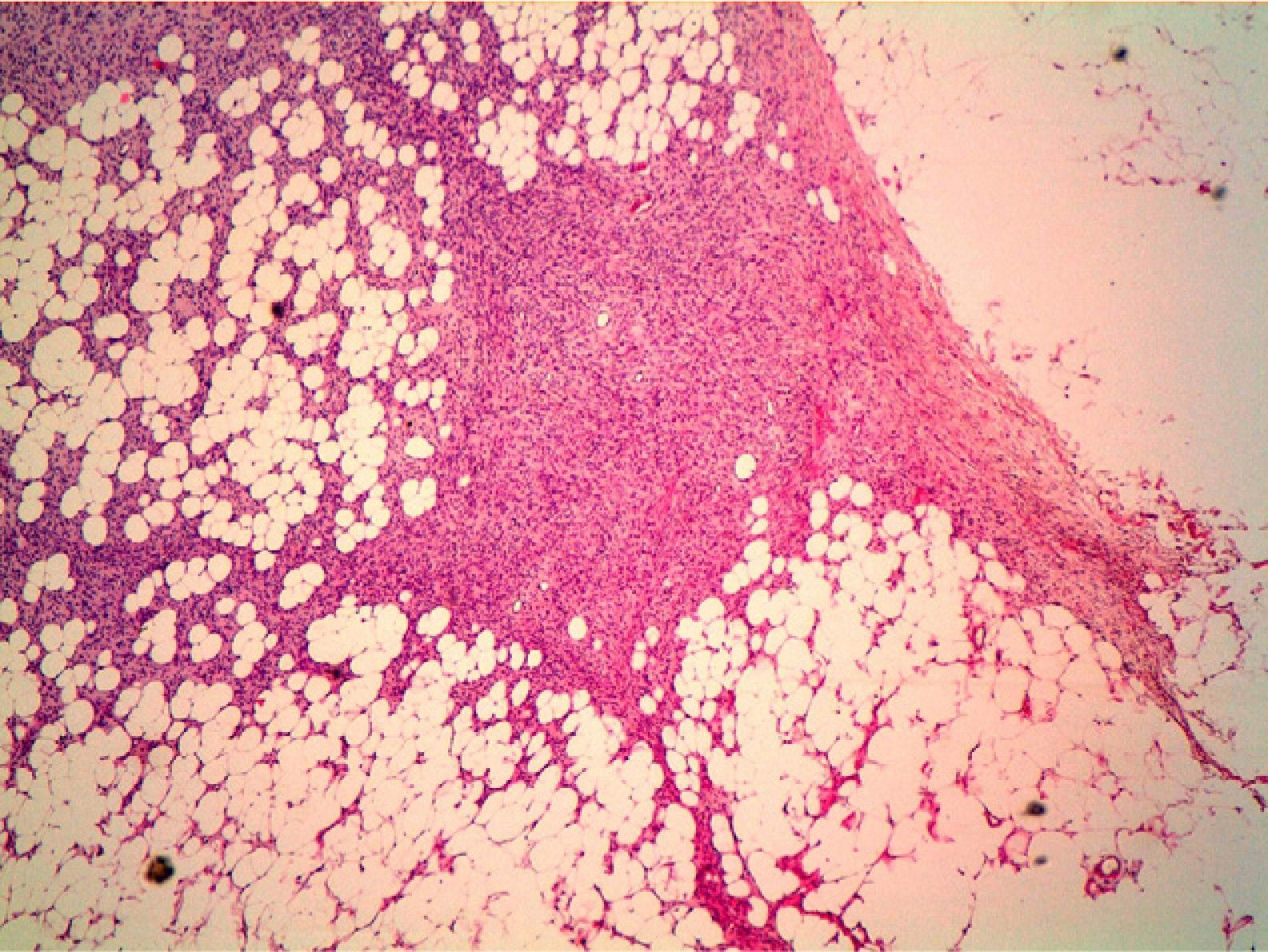
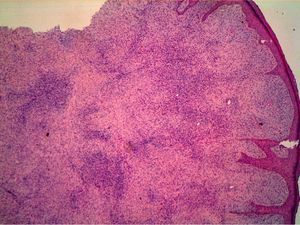
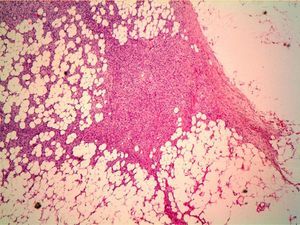
HistopathologyHistopathology shows a lesion with a tumor-like appearance, originating in the dermis, and that invariably invades subcutaneous cell tissue in an irregular and diffuse fashion (Fig. 4). There are 2 patterns of invasion of deep-lying tissues, one a diffuse infiltration with islands of adipocytes among tumor fragments to give a honeycomb appearance and one a layered pattern (Fig. 5). The most characteristic features of this tumor are its pseudopod-like growths, which extend like tentacles from the sides and base up to 3cm or more from the macroscopic margin of the tumor.15 This pattern of invasion is the main reason for the high local recurrence rate and the difficulty in full excision of DFSP lesions. Occasionally, the fascia, muscle tissue, or bone tissue may be involved.
A DFSP lesion is composed of elongated spindle cells with a fairly homogeneous form, little pleomorphism, an elongated nucleus and scant cytoplasm. These cells are distributed in a storiform cartwheel or spiral pattern. Mitotic figures are usually present, but in small numbers (fewer than 5 mitotic figures per 10 high-power fields)15 and without atypia.3 There is usually a limited amount of stroma. The epidermis is often unaffected, although it is occasionally ulcerated, atrophic, or hypertrophic. The lesions are generally separated from the epidermis by a narrow band of spared dermis, particularly in the early stages. In neonates, this tumor is formed mainly from fusiform or ovoid cells which are small and have an immature appearance.7
DFSP lesions with a capacity for metastasis cannot be identified according to depth of invasion, mitotic index, cellularity, or nuclear atypia.15
Histologic variants reported include those with the presence of hypercellular areas without fibrosarcomatous characteristics; myxoid types; granular, atrophic, sclerotic types; variants with multinucleated cells present; and Bednar tumor or pigmented variants, which occur more frequently in blacks.15,25,30 These variants do not affect the prognosis of the patient.31Approximately 7% to 15% of tumors contain a fibrosarcomatous component, although this presentation is exceptional in childhood. Hypercellular areas can be observed, along with an increase in mitotic figures and clear arrangement of projections of spindle cells forming bundles that resemble a fibrosarcoma.31 In 50% of cases, staining for CD34 is negative.16 In general, it is thought that these fibrosarcomatous changes represent a form of tumor progression that is associated with more aggressive behavior than is usual in DFSP. Growth in such cases is faster and expansive with invasion of muscle tissue, the tumor is larger, the time from onset to diagnosis is longer, and immunoreactivity with p53 and ki67 is higher.32 These tumors have also been associated with a high recurrence rate and a higher risk of metastasis (by 10% to 15%). Fibrosarcomatous changes have been associated with incomplete excision that predisposes the residual tumor cells to develop molecular changes that allow them to acquire metastatic potential.30,33,34 Recently, studies have shown that if surgical excision is performed appropriately, leaving disease-free margins, the probability of local recurrence is similar to that of conventional DFSP.32,35 It is important that pathologists recognize and report these changes in the tumors analyzed, even if present in small proportions, as such findings may still be clinically relevant.33–36
Currently, giant-cell fibroblastoma is considered a different expression of the same neoplasm,37 or as a histologic variant of DFSP corresponding to childhood onset (although cases have been reported in adults).9,16,38,39 The clinical features of giant-cell fibroblastoma are very similar to those of DFSP, that is, the tumor is slow-growing and nodular, progressing to a protuberant appearance, and located essentially on the trunk (Fig. 6). The histopathologic study shows spindle cells arranged in a storiform pattern, along with multinucleated giant cells and characteristic pseudovascular spaces that seem to reflect a loss of cell cohesion.30 The immunohistochemical and cytogenetic findings are also the same. Like DFSP, this tumor has a high local recurrence rate, although metastases have not been reported until now.38 Hybrid lesions, with features of both DFSP and giant-cell fibroblastoma, have also been reported.7,40
It is clearly difficult to perform a histologic differential diagnosis of DFSP with other lesions, and in particular with fibrohistiocytic tumors. DFSP should be distinguished from neurofibroma, dermatofibroma, hemangioma, fibrosarcoma, malignant fibrous histiocytoma, and myxoid liposarcoma, among other entities.3,37
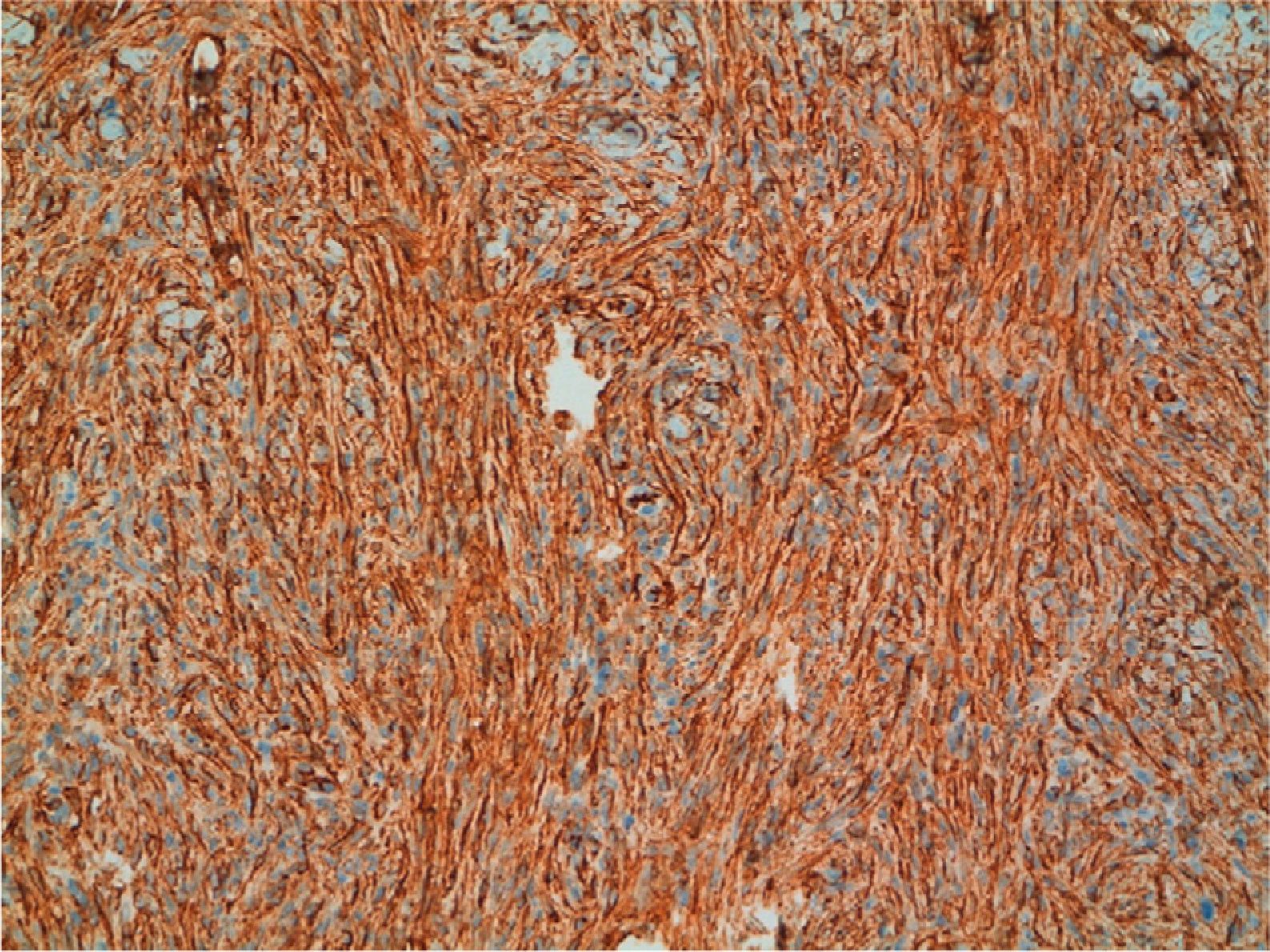
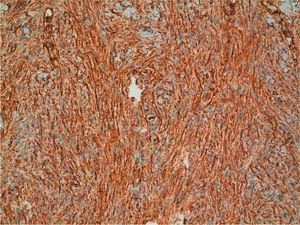
Positive immunostaining for CD34 and vimentin (Fig. 7) and negative immunostaining for S100, factor XIIIa, desmin, and smooth muscle actin can confirm a diagnosis of DFSP. Conventional dermatofibroma, unlike DFSP, stains negative for CD34, while tenascin and factor XIIIa stains are positive.30 New markers that are positive in DFSP and negative in dermatofibroma (namely, apolipoprotein D)41 and positive in dermatofibroma and negative in DFSP (namely, stromelysin 342 and CD163) have been proposed.43 Finally, it is worth pointing out that not all CD34+ spindle cell proliferations in the dermis indicate the presence of DFSP, as other sarcomas and even some benign fibrohistiocytic lesions are also positive.44 Recently, a new congenital entity has been described in children, denoted dermal dendrocytic hamartoma.9,45 The cells are positive for CD34, but COL1A1-PDGFB is not expressed according to molecular studies.
An alternative method for confirming the diagnosis of DFPS is staining for platelet-derived growth factor receptor β (PDGFRB), thereby demonstrating overexpression of this molecule on the surface of tumor cells, although the usefulness of this approach has yet to be demonstrated.31
Cytogenetic StudiesDFSP is characterized in 70% to 90% of both adults and children by the presence of a characteristic cytogenetic abnormality consisting of a supernumerary ring chromosome or translocation between chromosomes 17 and 22. In both cases, there is fusion (17q22) (22q13) of the COL1A1 gene (α1 chain of type 1 collagen) of chromosome 17 with the PDGFβ gene (β-chain of platelet derived growth factor) of chromosome 22. PDGFB codes the β-chain of PDGF, a ligand of the PDGF tyrosine kinase receptor located on the cell surface. In children, translocations are more common, and supernumerary ring chromosomes have not been detected to date.7,9,15,21,28
PDGFB is a growth factor that acts as a potent mitogen for connective tissue cells.16 The product of the COL1A1-PDGFB fusion gene induces tumor formation through increased expression of PDGFB in tumor cells, leading to autocrine or paracrine stimulation of the tumor, by activation of the PDGFB receptors. This molecular abnormality is essential for the development of DFSP. The product of the COL1A1-PDGFB fusion gene can be detected in samples embedded in paraffin by PCR techniques and fluorescence in situ hybridization (FISH) analysis. This translocation is also present in Bednar tumor and in giant-cell fibroblastomas.7
In all cases studied, translocation between chromosomes 17 and 22 leads to chimeric COL1A1-PDGFB RNA, in which exon 1 of PDGFB has been eliminated and substituted by a variable segment of the COL1A1 gene, from exon 7 to exon 47. This might indicate that COL1A1 sequences play a secondary part in coding the final protein, and that they are not essential for tumor growth, unlike PDGFB sequences that allow the synthesis of the corresponding mature protein and so would take on a key role.30,46 No association has been found between the different COL1A1-PDGFB fusion products and patient and clinical characteristics (age, sex, tumor size, and tumor site) or the histologic subtype in the patients studied.44
In a minority of cases (8%), these abnormalities were not detected.9,13,15,44,47,48 This suggests that other genes, so far thought to be located on chromosomes 5, 7, 8, and 21, may participate in this tumor process.30 In children, a reciprocal translocation 46, XX, t (X,7) (q2112;q11.2) has also been reported.48
Diagnosis and StagingIn diagnosis, DFSP is suspected from the clinical characteristics, although in some cases, diagnosis might be extremely difficult as this is a very rare tumor, the clinical features are not striking, and its course is slowly progressive. The diagnosing physician should perform a complete examination of all the skin of all patients diagnosed with DFSP.
Definitive diagnosis requires histopathologic study with immunohistochemical staining. When there is strong clinical suspicion but the initial biopsy results are inconclusive, a repeat biopsy is recommended.36 Cytogenetic studies can provide confirmation of diagnosis, although in 8% of cases, these studies are negative.
To establish a diagnosis of tumor recurrence, biopsy of the area or fine needle aspiration should be performed.16
Magnetic resonance imaging of the affected area is used to define the extension of the tumor and invasion of different tissues, and to monitor for recurrences.21,29,49 However, it does not seem useful for detecting residual tumor in cases of recent incomplete excision or for defining the side margins of the tumor.50
Ultrasound can be useful in selected cases. Computed tomography is not indicated unless bone involvement or lung metastasis is suspected.16,39
Given that lymphatic and blood-borne dissemination is uncommon, tracking of tumor metastasis is not recommended as a complementary test unless there are clinical findings to suggest tumor spread. Elective regional lymph node dissection is not indicated as part of the management of DFSP. However, clinical examination of the nodes is important in the follow-up of these patients.15,16
A definitive staging system for DFSP has not been described, although according to a German clinical scale, stage I corresponds to a primary localized tumor, stage II to a tumor with lymph node metastasis, and stage III to a tumor with distant metastasis.51
Outcomes and Follow-UpRecurrence is less frequent in children (9%).6 Jafairan et al.15 did not observe any local recurrences in 8 children (mean follow-up, 5 years).
Recurrence is most common in patients with fibrosarcomatous changes, positive microscopic margins, increased cellularity, high mitotic figures, or older than 50 years.21,30,52 The tumor size does not seem to have a significant influence on recurrence or prognosis.53 It is also important to remember that the main predictor of recurrence is incomplete excision of the lesion.12,33
Most recurrences present within 3 years of surgery, although recurrence as long as 26 years after excision of the primary tumor has been reported.54 Thus, long-term follow-up of these patients is required.
Metastasis is uncommon, occurring in 3% to 5% of cases, and is usually preceded by multiple local recurrences, although metastasis has been reported without any prior recurrence.55 In the largest pediatric series, of 166 patients, 1 child died of progressive local recurrence.6 A case of lymph node metastasis in a patient aged 21 years and a case of lung metastasis in a patient aged 31 years after childhood DFSP have been reported.56,57 McKee and Fletcher14 and Thornton et al.29 did not find any recurrences or metastases in 2 series of 8 and 10 patients, respectively, in which the mean follow-up periods were 13 and 36 months, respectively.
The appearance of metastasis is a sign of poor prognosis, with a mean survival of 2 years.12,58 Metastasis is usually to the lungs; fewer than 1% of metastases are to lymph nodes, so systematic lymph node dissection is of little value in the management of this tumor.15
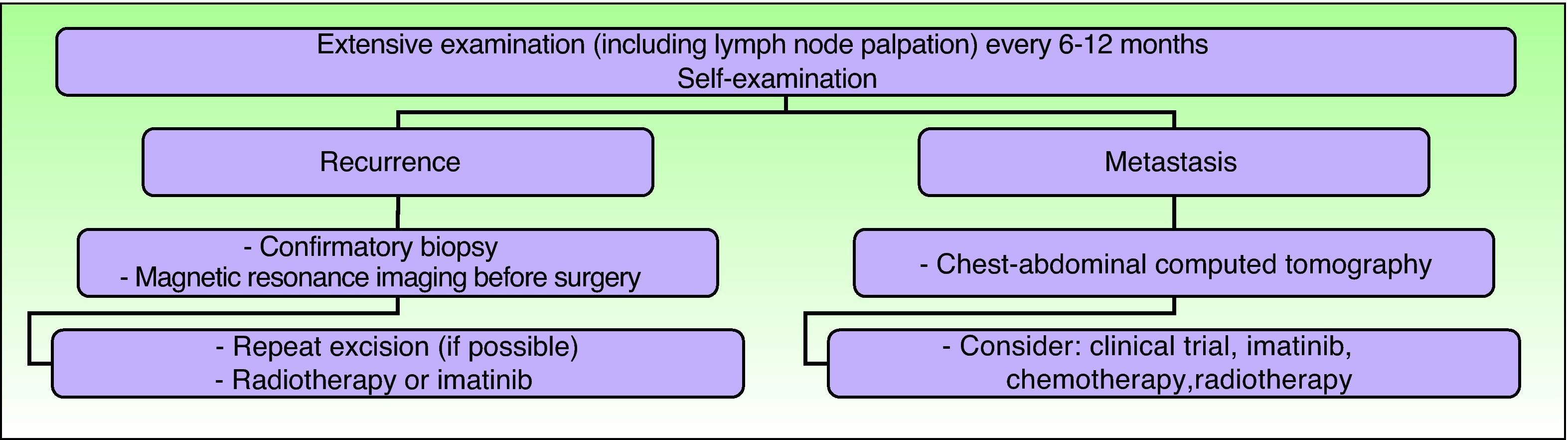
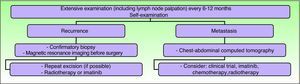
Clinical follow-up is required every 6 to 12 months, particularly in the first 3 years after surgery, and should include palpation of the surgical scar and locoregional lymph nodes.5,36 Some authors are in favor of lifelong follow-up.15,59 Any abnormal scarring should be considered as a potential recurrence, and should be biopsied as soon as possible.15 The need for regular examinations of the scar should be explained to the patient and parents. Routine imaging studies are not necessary during the follow-up of these patients, although if a recurrence is suspected, magnetic resonance imaging is the most useful type of study as it helps establish the extension of the tumor and guide subsequent treatment (Fig. 8).
Clinical follow-up. Adapted from the 2010 National Comprehensive Cancer Network practice guidelines in oncology.53
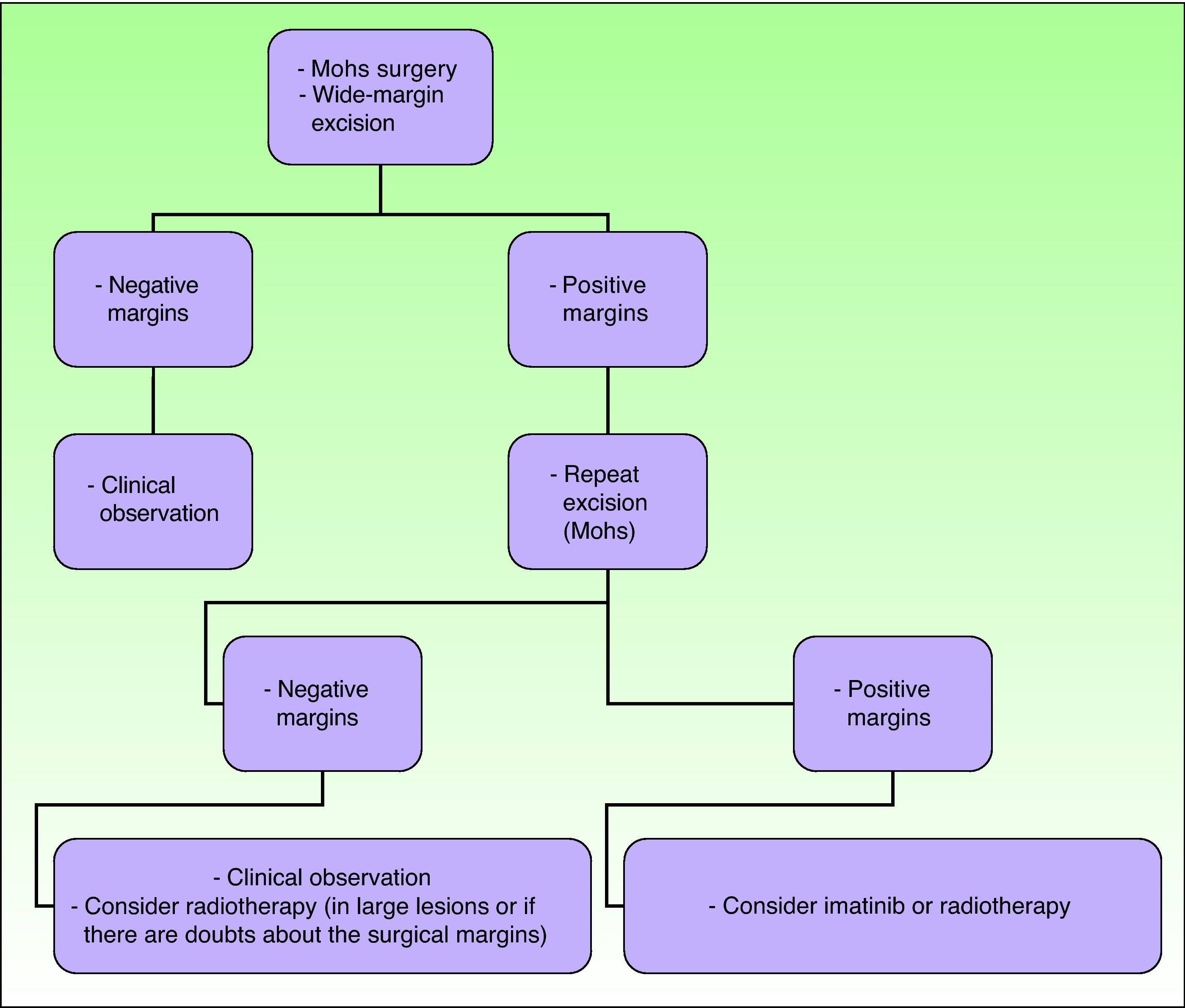
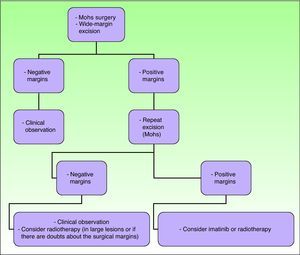
The main objective of treatment in these patients is to completely excise the lesion, because such excision is the strongest positive prognostic factor, associated with very low relapse rates and survival rates close to 100%.2,16 Recurrence rates after simple excision range from 33% to 60%. If wide margins are also taken, recurrence rates fall to between 10% and 30%, and if Mohs micrographic surgery is used the recurrence rates fall farther to between 0% and 6.6%.21
The 2010 National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology summarize the current recommended approach to DFSP, although they are not specific for the pediatric population (Fig. 9).36
Treatment of dermatofibrosarcoma protuberans. Adapted from the 2010 National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology.53
In recent decades, the use of Mohs micrographic surgery has been encouraged. The recurrence rates with this technique have decreased to between 0.6% and 6%, and so it has become established as the technique of choice for DFSP.29 It is particularly important in tumors with poorly defined margins, in sites on the head, neck, acral regions, the chest (particularly in children), and in tumor recurrences. Thornton et al.29 saw no recurrences in a series of 10 children treated using this technique. Snow et al.59 reported a series of 29 patients of all ages without any local recurrences at 5 years, although in these authors’ review of the literature on 136 patients with a minimum follow-up of 5 years, the recurrence rate was 6.6%. Essentially, the advantages of this technique lie in the lower number of surgical procedures, the smaller scar size, and the greater sparing of healthy tissue, with both cosmetic and functional benefits.13,60
The use of this technique in children has some limitations. First, no randomized, controlled trials have compared conventional surgery to Mohs micrographic surgery in patients of this age group.3 In a retrospective study, Love et al.13 compared to the use of conventional surgery with wide margins and Mohs micrographic surgery in 61 cases of congenital DFSP. They reported 100% tumor excision rates with Mohs micrographic surgery and 89% excision rates with conventional surgery, and so they recommended the Mohs technique. Iqbal et al.,5 in a review of 15 cases in patients under 18 years of age, observed no recurrences after 4 years of follow-up in patients treated with conventional excision with wide margins or Mohs micrographic surgery. An added difficulty in performing this technique in children is the need to perform multiple passes in patients who are under general anesthetic.3
When Mohs micrographic surgery cannot be performed, wide margins should be taken when excising and the procedure repeated until histologically negative margins are obtained. It is not clear what margins are required in children to achieve local tumor control, as the level of evidence is low.2 Some authors establish a margin of 3cm, including muscle fascia, in all children aged more than 5 years. For younger children, including neonates, tumor-free margins of 1cm seem to be reasonably sufficient.7 When Kimmel et al.61 investigated resection margins in DFSP, they concluded that the macroscopic size of the tumor is a poor indicator of the true histologic extension. Other authors, in a review of 66 DFSP cases, found the local recurrence rate was around 47% when margins were less than 3cm and around 7% when margins were between 3 and 5cm.12
At sites where space is a problem, such as the head, neck, and acral regions, wide-margin excision is not always possible in view of substantial cosmetic defects and functional repercussions secondary to excision; in these cases, the recurrence rates range from 50% to 75%.3 When margins are possible, and surgery cannot be repeated, postoperative radiotherapy or imatinib is indicated, as we shall see below.2
Advancement flaps or grafts are required to cover the surgical defect left after excising extensive lesions. In such cases, it is important to be certain that the margins are negative, as advancement flaps may hinder early detection of a local recurrence.2 If it is impossible to demonstrate negative surgical margins, debridement should be avoided and a total skin graft would be preferred to facilitate early detection of recurrence.36
Molecular TherapyIn light of the recent identification of the importance of PDGFB-PDGFRB signaling in the pathogenesis of DFSP, investigators have explored the utility of imatinib (Glivec), a selective inhibitor of the tyrosine kinase transmembrane receptor of several proteins, such as PDGFRB, in the treatment of these tumors. Imatinib is approved as a first-line therapy for chronic myeloid leukemia and metastatic or inoperable gastrointestinal stromal tumors, which also present aberrant tyrosine kinase activation.16
Imatinib was recently approved by the US Food and Drug Administration for the treatment of adult DFSP; the indications are for inoperable, recurrent, or metastatic tumors.62 Imatinib can also be used preoperatively to shrink the tumor so that the excision required is smaller. The dosage ranges from 400 to 800 mg a day, taken orally, and side effects are limited, although both the dosing and duration of therapy are still subject to debate.6 The known side effects of this drug include dyspepsia, nausea, vomiting, immunosuppression, bleeding, elevated liver enzymes, and renal failure.8
About 65% of adult patients with DFSP tumors have an overall response to imatinib.16 Lack of response is related to mutations that induce resistance in PDGFRB.63 Some tumors without this translocation and those that do not depend on PDGFRB signaling likewise do not respond.64 It is therefore necessary to request a cytogenetic study of the tumor before making a treatment decision.16,36
Some authors have used this agent in children, although it is currently not approved in patients under 18 years of age, and pharmacokinetic data in this age group are not available. However, there seems to be sufficient data from phase I and II trials in chronic myeloid leukemia to enable appropriate dosing in children.59,65 The dosages used are 400 to 500 mg/m2/d. In pediatric case reports, no adverse effects of imatinib have been reported.6 The tablets have a bitter taste, and so rectal administration may be an alternative.66
Price et al.8 gave imatinib to an 18-month-old patient with congenital DFSP, and reported that the response after 23 weeks was good according to magnetic resonance imaging. However, no details of the long-term outcome were given. Gooskens et al.6 reported 3 more cases of treatment with imatinib in infants aged 6 to 12 months. They achieved significant reduction in tumor size prior to excision.
Gooskens et al.6 highlighted the usefulness of molecular screening to detect the COL1A-PDGFB abnormality in surgical margins, as although these may be histologically negative, areas with microscopic tumor remnants may persist. If molecular screening is positive, imatinib could be used as a coadjuvant to reduce the risk of recurrence. We had the opportunity use this approach ourselves in an 8-year-old boy. Margins were histologically negative, but translocation was detected in one of them (in paraffin samples using FISH analysis). Extended margins were all histologically and cytogenetically negative, and the patient is disease-free after 11 months (unpublished data). Larger prospective studies are needed to determine the clinical value of cytogenetic analysis of the tumor margins to detect minimal residual disease, but undoubtedly in children it could be a useful tool for guiding therapeutic decisions after surgery.6
RadiotherapyAlthough radiotherapy has been employed first-line,67 it is more often used as an adjuvant to surgery in cases of excision with positive margins, and/or if fibrosarcomatous changes are present.68 Postoperative radiotherapy should be considered if lesions are large or if there is a lack of certainty regarding the adequacy of surgical margins. In lesions with positive margins or those with an anatomical limitation close to the surgical margin, doses of 5000 to 6000 cGy are applied in 200-cGy fractions per day. If clinically viable, the field is extended by 3 to 5cm beyond the surgical margin.36
ChemotherapyIn certain cases, chemotherapy can play an important role. One case report described a patient with local recurrences who responded to a combination of methotrexate and vinblastine.69
Isolated hyperthermic limb perfusion has been performed with melphalan and TNF-α to reduce the tumor size prior to surgery.70
In patients with metastasis, chemotherapy, radiotherapy, excision, or imatinib should be considered.16 Alternatively, patients could be considered for inclusion in a clinical trial.16
ConclusionsDiagnosis of DFSP in childhood is a challenge and requires a high degree of clinical suspicion. Both congenital tumors and those acquired during childhood may be confused with other benign lesions such as hemangiomas and vascular malformations for a long time. Thus, any poorly-defined, slow-growing plaque or nodule of uncertain diagnosis should be biopsied. Diagnostic confirmation usually requires immunohistochemical techniques, and it is also possible to perform cytogenetic studies to detect the COL1A-PDGFB abnormality.
The behavior of this tumor in children is usually less aggressive and recurrence and metastasis rates are lower than those described for adults in the literature.
The main aim of treatment of DFPS is to completely eradicate the tumor. If the tumor is large or poorly defined, magnetic resonance imaging before surgery can help determine the extension and exact size and facilitate appropriate planning. Mohs micrographic surgery is the technique of choice in DFSP and is particularly useful for sites such as the head, neck, and acral areas, allowing less aggressive resection and reducing the incidence of local recurrence.
Radiotherapy or imatinib may be considered in initial management in children with inoperable tumors as a way to reduce the size prior to surgery and in those cases with recurrent or metastatic lesions.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank Dr. Teresa Aramendi for her invaluable help with the histologic images.
Please cite this article as: Valdivielso-Ramos M, Hernanz JM. Dermatofibrosarcoma protuberans en la infancia. Actas Dermosifiliogr. 2012;103:863–73.