Calcinosis cutis (CC) is defined as the deposition of calcium salts in the skin. The condition is divided into 5 types: calciphylaxis and dystrophic, metastatic, idiopathic, and iatrogenic CC. Dystrophic CC is the most common form and usually occurs in association with autoimmune diseases. CC can be treated surgically or with the use of drugs such as diltiazem, bisphosphonates, warfarin, ceftriaxone, probenecid, minocycline, or aluminum hydroxide. Calciphylaxis is defined as calcification of the media of small- and medium-sized blood vessels in the dermis and subcutaneous tissue. Clinically, calciphylaxis causes livedo racemosa, which progresses to retiform purpura and skin necrosis. First-line treatment is with sodium thiosulfate. We present a review of the calcifying disorders of the skin, focusing on their diagnosis and treatment.
La calcinosis cutis (CC) se define como el depósito de sales de calcio en la piel. Esta entidad se clasifica en 5 tipos que incluyen la CC distrófica, metastásica, idiopática, iatrogénica y calcifilaxis. La calcificación distrófica constituye el tipo más frecuente y aparece principalmente en enfermedades autoinmunes. El tratamiento de la CC incluye la extirpación quirúrgica o el uso de fármacos como diltiazem, bifosfonatos, warfarina, ceftriaxona, probenecid, minociclina e hidróxido de aluminio. La calcifilaxis se define como la calcificación de la capa media de vasos de pequeño y mediano tamaño de la dermis y tejido celular subcutáneo. Clínicamente se manifiesta como un síndrome de livedo racemosa que progresa a púrpura retiforme y necrosis cutánea. La primera línea de tratamiento es el tiosulfato sódico. El objetivo de esta revisión es proporcionar un análisis de los diferentes trastornos de calcificación cutánea enfocada en su diagnóstico y tratamiento.
Calcinosis cutis (CC) is caused by the deposition of insoluble calcium salts in the skin. Associated diseases, serum calcium and phosphorus levels, and underlying cause are used to classify the entity as dystrophic CC, metastatic CC, idiopathic CC, iatrogenic CC, or calciphylaxis (Table 1).1,2 Other less common forms of CC, which can be indistinctly classified as dystrophic or idiopathic, include CC circumscripta, CC universalis, tumoral calcinosis, and transplant-associated CC.
Classification of Calcinosis Cutis by Pathogenesis, Associated Diseases, and Levels of Serum Calcium and Phosphorus.
| Type of Calcification or Calcinosis Cutis | Pathogenesis | Associated diseases | Serum Calcium and/or Phosphorus Levels |
|---|---|---|---|
| Dystrophic calcification | Secondary to tissue damage | Autoimmune diseasesSystemic sclerosisDermatomyositisLupus erythematosus and lupus panniculitisOverlap autoimmune syndromes of connective tissueUndifferentiated connective tissue diseaseMixed connective tissue diseaseSkin neoplasmsPilomatrixomaTrichilemmal cystCollagen or elastic fiber diseasesElastic pseudoxanthomaWerner syndromeEhlers-Danlos syndromeInfectionsOnchocercosisCysticercosisHistoplasmosisCryptococcosisIntrauterine herpes simplexTraumaOthersPorphyria cutanea tardaPancreatic panniculitis | Normal |
| Metastatic calcification | Calcium precipitation in the skin | Chronic kidney failureHyperparathyroidismHypervitaminosis DSarcoidosisMilk and alkaline syndromeMalignant neoplasms | Abnormal |
| Idiopathic calcification | Unknown. No previous damage to skin or metabolic disturbances | Tumoral calcinosisCalcified subepidermal nodules (nodular calcinosis of Winer)Scrotal calcinosis | Normal |
| Iatrogenic calcification | Side effect of medical treatment | Intravenous calcium-containing solutionsVenipuncture sites | Normal |
| Calciphylaxis | Calcification of the small vessel walls in the dermis and subcutaneous cell tissue with subsequent ischemia | Chronic kidney failureOther nonuremic causes | Abnormalities can be observed |
CC and calcification are used indistinctly as synonyms. Calciphylaxis will be treated in a separate section given its different clinical presentation and pathophysiology.
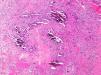
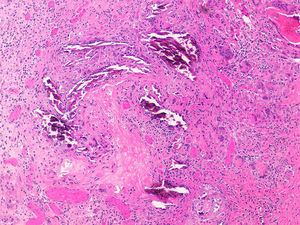
Pathogenesis and Types of CCDystrophic CalcificationDystrophic calcification is the most frequent type of CC. Serum calcium and phosphorus levels are normal. The condition is caused by damage or alteration to collagen, elastin, or subcutaneous fat.1 Tissue damage, hypoxia, or hypovascularization induces the release of phosphate-binding proteins by necrotic cells, and these proteins take up phosphate thereby resulting in calcification. Proinflammatory cytokines such as interleukin (IL) 6, IL-1β, and tumor necrosis factor also play an important part.3–5 The calcified deposit is composed of hydroxyapatite and amorphous calcium phosphate.6 In the pathology studies, the calcium deposits have a dark blue color with hematoxylin-eosin staining (Fig. 1) and a blackish tone with Von Kossa staining. Foreign body reactions and fibrosis can appear around the calcium deposits.4
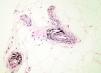
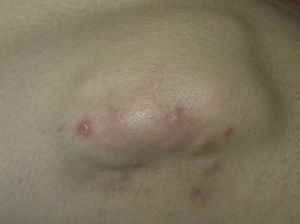
This type of calcification is associated with a range of diseases that lead to connective tissue damage. These diseases include autoimmune diseases, hereditary connective tissue diseases, skin neoplasms, panniculitis, and infections.1 The main autoimmune diseases associated with dystrophic calcification are systemic sclerosis, dermatomyositis, and lupus erythematosus (Table 2). Patients with overlap syndromes and mixed and undifferentiated connective tissue diseases rarely develop dystrophic CC. Other diseases in which dystrophic calcification has been reported include rheumatoid arthritis, morphea-like or linear localized scleroderma, and primary Sjögren syndrome.3,7 The appearance of dystrophic calcification in porphyria cutanea tarda is uncommon, and tends to occur in those patients with scleroderma-like lesions. Calcification is initiated by repeated episodes of local inflammation, blister formation, and fibrosis that occur in the skin in this type of porphyria. These calcium deposits are localized in the periarticular regions of the elbows and knees, and also on the backs of the hands, scalp, preauricular region, and neck.8 Among the hereditary diseases of the connective tissue associated with dystrophic calcification, elastic pseudoxanthoma, Werner syndrome, and Ehlers-Danlos syndrome are of particular note.9–12 In the case of neoplasms, pilomatrixoma also known as Malherbe calcifying epithelioma, and trichilemmal cyst deserve mention. Pilomatrixoma presents in approximately 75% of cases of dystrophic calcification. In the pathology study, calcification normally settles in the intracellular space as granules within ghost cells (Fig. 2). In trichilemmal cysts, foci of calcification can be observed in approximately 25% of cases.1,2 Dystrophic calcification may also develop in basal cell carcinoma, desmoplastic trichoepitheliomas, and other mesenchymal tumors.1 Pancreatic and lupus panniculitis are more frequently associated with this type of calcification.1,13 Some exotic diseases are also associated with dystrophic calcification, such as, for example, onchocercosis or river blindness, cysticercosis, histoplasmosis, and cryptococcosis. Finally, cases of calcification have been observed arising from trauma or burn scars.1
Dystrophic Classification in Autoimmune Diseases of Conjunctive Tissue.
| Disease | Systemic Sclerosis | Dermatomyositis | Lupus Erythematosus |
|---|---|---|---|
| Frequency | 25% | Adult dermatomyositis: 20%Juvenile dermatomyositis: 44%-70% | Rare |
| Time since diagnosis | More than 10 years. Although it can appear even before diagnosis | Adult dermatomyositis: 8 years approximately after diagnosisJuvenile dermatomyositis: 2-3 years after diagnosis | Course of more than 20 years. |
| Predominant site | Hands and feetLimbsRegions of microtrauma | LimbsTrunk | LimbsButtocks |
| Characteristics | Mainly in patients with limited systemic sclerosis and positive for anticentromere antibodies | Juvenile dermatomyositis can lead to calcinosis universalis forming an exoskeleton.Early and potent treatment reduces the incidence of calcinosis in juvenile dermatomyositis | Normally consists of a chance radiological findingLupus panniculitis can also progress to dystrophic calcinosis, normally 5 years after diagnosis |
Metastatic calcification is characterized by abnormal levels of serum calcium and/or phosphorus resulting in precipitation of calcium salts in normal tissue with no structural damage. When the abnormal serum levels return to normal, the lesions usually remit. The degree of hyperphosphatemia will determine the number and size of these calcium deposits. Metastatic calcification is usually located in periarticular regions. The most frequent cause of metastatic calcification is chronic kidney failure (CKF) (Fig. 3). Other causes of metastatic calcification include hypervitaminosis D, hyperparathyroidism, sarcoidosis (production of vitamin D by the sarcoidosis granulomas), milk and alkali syndrome (excessive consumption of antacids or calcium-containing food), and malignant neoplasms (destructive metastatic or paraneoplastic mechanism).1,2,14
Idiopathic CalcificationIdiopathic CC refers to calcinosis without damage to underlying tissue, unlike dystrophic calcification, and without abnormal calcium or phosphate metabolism, typical in metastatic calcification. There are 3 main forms, familial tumoral calcinosis, subepidermal calcified nodules, and scrotal calcinosis.1
Familial tumoral calcinosis includes several orphan diseases of recessive inheritance that are normally associated with an increase in phosphate resorption in the proximal tubule of the kidney. Clinically, these conditions become manifest through the formation of periarticular calcium masses and masses in the acral regions of young patients.9,15 Given its association with hyperphosphatemia, some authors consider this condition as a form of metastatic calcification.1 The subepidermal calcified nodules or Winer nodular calcinosis appear in children and can even be observed at birth. Clinically, the nodules present as hard, solitary white-yellowish papules, mainly on the head and limbs. Finally, scrotal calcinosis constitutes the third entity of idiopathic CC.1 In these patients, nodules of different sizes, numbers and forms are observed. They are readily palpable and marble-like. Normally, lesions are asymptomatic, but some patients may report pruritus.16
Iatrogenic CalcificationIatrogenic calcification is caused by the therapeutic or diagnostic use of calcium- or phosphate-containing substances. The entity has been reported with intravenous use of calcium gluconate, calcium chloride, and even para-aminosalicylic acid for the treatment of pulmonary tuberculosis.1,17 It has also been observed after application of conductive pastes containing calcium chloride for placement of electrodes used in encephalograms.2 Other causes include chemotherapy-induced tumor lysis syndrome, and it has also been reported after organ transplantation.1
CalciphylaxisCalciphylaxis or calcifying uremic arteriopathy is an entity characterized by calcification of small and medium-sized vessels (calcifying vasculopathy) of the dermis and subcutaneous cell tissue. This may then lead to ischemia and necrosis of the affected skin.18 Calcium-phosphorus metabolism can be normal or abnormal.1 This entity essentially affects patients with terminal CKF who undergo hemodialysis or peritoneal dialysis, or who are recipients of kidney transplants, with a prevalence of 1% to 4% in these patients.19 The mean age of onset is 48 years, and incidence is higher in women and Caucasians.20,21 With regards the pathophysiology of calciphylaxis, hyperphosphatemia and toxins produced in patients with CKF lead to an increase in reactive oxygen species (oxidative stress) and an inflammatory response with release of proinflammatory cytokines such as IL-6, IL-1, and tumor necrosis factor. This oxidative stress and the inflammatory processes decrease the concentration of local calcification inhibitor proteins, such as circulating glycoprotein fetuin-A (α2-Heremans-Schmid glycoprotein) and the matrix gla protein, as well as expression of osteogenic differentiation genes in smooth muscle cells in the vessels. These phenomena lead to vascular calcification of the medial layer and development of endovascular fibrosis, hyperplasia of the intima, and vascular thrombosis, all of which are responsible for subsequent ischemic processes.22 Traditionally, calciphylaxis has been classified as a type of metastatic calcification in which there is a disturbance of the calcium and phosphate levels in blood. However, a considerable number of patients with calciphylaxis do not have renal damage or have normal levels of calcium and phosphate in blood.23 There are a series of risk factors for the development of calciphylaxis in patients with CKF such as hyperparathyroidism; calcium-phosphorus product>70; vitamin D treatment; diabetes mellitus; arterial hypertension; female sex; obesity; warfarin treatment; S or C protein deficit; immunosuppression; liver disease; hypoalbuminemia, weight loss or malnutrition; congestive heart failure; and presence of arteriovenous fistula.24,25 Nonuremic causes of calciphylaxis have also been reported. These include primary hyperparathyroidism; malignant neoplasms (cholangiocarcinoma, chronic myeloid leukemia, melanoma, metastatic breast cancer, multiple myeloma); chronic liver disease; diabetes mellitus, autoimmune diseases (giant cell arteritis, rheumatoid arthritis, systemic lupus erythematosus, and Crohn disease); protein S or C deficiency; antiphospholipid syndrome; weight loss; drugs (corticosteroids, high doses of vitamin D and analogues, chemotherapy); polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS); and nonterminal CKR.23
Calciphylaxis is associated with a high mortality, ranging from 46% to 80% of cases. The most frequent complication and subsequent cause of death is sepsis due to infection of necrotic ulcers.26
Clinical Manifestations and Complementary Tests in Patients With Suspected CC (Excluding Calciphylaxis)Most CC lesions develop gradually and are asymptomatic. Clinically, these lesions can range from localized and asymptomatic nodules to forms that involve large areas of the body surface, leading to muscular atrophy, joint contracture, and cutaneous ulceration that may be associated with secondary infections as an additional complication.3,7
For a patient with CC, a series of laboratory tests and imaging studies are recommended to identify the type of calcification and determine its etiology. In all patients, and in order to classify the type of CC, serum calcium, inorganic phosphate, alkaline phosphatase, and albumin levels should be measured. When the calcium-phosphorus metabolism is disturbed, the levels of calcium and phosphorus are often normal because parathyroid hormone is elevated.1 Ideally, ionized calcium should be measured, but problems with sample processing and the high cost of the technique are a barrier to widespread use. When total calcium is measured, it is recommended to adjust for albumin (or plasma protein) levels, given that calcium binds strongly to proteins.
Once we have classified the type of CC, we can conduct a series of complementary tests to determine the etiology (Table 3).
Additional Tests To Determine Etiology in Patients With Calcinosis Cutis.
| Additional Test | Etiologic Information |
|---|---|
| Hematology | Lupus erythematosusMalignant neoplasms |
| Biochemistry with creatinine and urea | Chronic kidney failure |
| Parathyroid hormone (PTH) | Hyperparathyroidism |
| Vitamin D levels | Hypervitaminosis D |
| Calcium, phosphate, total proteins, albumin, 24h urine excretion of calcium/inorganic phosphate | * Metastatic calcification |
| Muscle enzymes: CPK, LDH, GOT, GPT, and aldolase | Dermatomyositis |
| ANA, anti-ADN, and anti-ENA | Lupus erythematosusSystemic sclerosis |
| Bicarbonate and arterial pH | Milk and alkaline syndrome |
| Imaging tests | |
| X-ray | Site and extension of calcification |
| Skin ultrasound | Hyperechoic image, occasionally with posterior acoustic shadow. Can determine the thickness, longitudinal extension, and site of calcification |
| Bone scintigraphy | More sensitive than X-ray. Useful for assessing calcification of nonvisceral soft tissue |
| Computed tomography (CT) and magnetic resonance imaging (MRI) | CT: Rarely used. It can identify visceral and nonvisceral calcification. Useful for detection of malignant neoplasmMRI: of limited use for assessing calcified structures |
| Skin biopsy | |
| Definitive diagnosis of calcinosis cutis | Hematoxylin eosin: dark blue hueVon Kossa stain: black hueForeign body reaction and fibrosis |
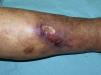
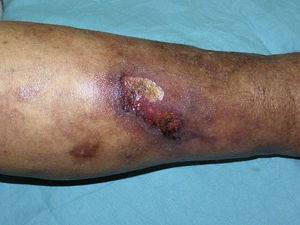
The skin lesions of calciphylaxis are located mainly in areas with greatest adipose tissue, such as the abdomen, thighs, lateral and posterior region of the legs, and buttocks.27 We differentiate between distal and proximal forms according to whether the site is below or above the knees or elbows, respectively. The proximal form has a worse prognosis and is usually associated with diabetes mellitus and greatest disturbance in calcium-phosphorus metabolism.18 Clinically, livedo racemosa and retiform purpura are observed and these may progress to skin necrosis, with the appearance of very painful ulcers on the affected skin (Fig. 4).28 Subcutaneous nodules, blisters, and ecchymosis may also appear. These skin ulcers may penetrate the fascia and lead to mutilation of areas such as the fingers and toes and even the penis. One characteristic of calciphylaxis is extreme ischemic pain resulting from skin infarction. Calciphylaxis may cause inflammatory myopathy with rhabdomyolysis, even without skin involvement. Other organs that might be affected include the heart, lungs, intestine, pancreas, and eyes. It is important to note that calciphylaxis lesions are associated with a high risk of infection.1,18
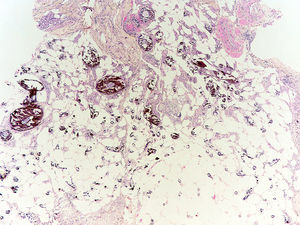
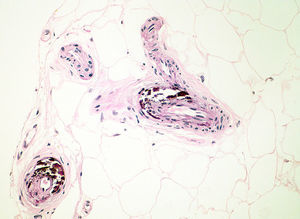
Diagnosis of calciphylaxis is based on the medical history, physical examination, and complementary tests for differential diagnosis with other diseases that cause vascular obstruction of cutaneous microcirculation.26,28 In the medical history, we will assess the risk factors described, mainly terminal CKF requiring hemodialysis. The presence of livedo racemosa syndrome, retiform purpura, and painful skin necrosis or ulcers are of note in the physical examination. Skin biopsy is the gold standard, but care should be taken and the overall health of the patient should be taken into account due to the risk of infection and problematic wound healing.18 Normally, a 4-6mm punch biopsy of subcutaneous cell tissue is taken from the middle of the eschar. However, it may be necessary to perform a more extensive incisional biopsy to observe calcification in the walls of the deepest vessels of the subcutaneous cell tissue.26 Histological study shows calcification of the medial layer of the small and medium-sized vessels in the dermis and subcutaneous cell tissue (Fig. 5). In addition, intravascular fibrin thrombi, subintimal fibrosis, inflammatory infiltrate, and endothelial damage can be seen without signs of vasculitis.19 The main differential histologic diagnosis should be performed with medial calcific sclerosis also known as Mönckeberg sclerosis, in which calcification occurs in the medial smooth muscle layers of the arteries and is not associated with changes in surrounding subcutaneous tissue or clinical complications. Calciphylaxis can also be associated with panniculitis, with fibrosis and lobular necrosis.26
Three clinical criteria have been proposed. When these are fully met, calciphylaxis can be diagnosed without the need for skin biopsy (Table 4).18
Criteria for Diagnosis of Calciphylaxis.
| Clinical Criteria | Pathology Criteria |
|---|---|
| Patient with chronic kidney failure in hemodialysis or with glomerular filtration rate less than 15mL/min/1.73m2 | Necrosis and ulceration of the skin with calcification of the medial layer and internal elastic membrane of small and medium-sized arteries of the dermis and subcutaneous cell tissue |
| Presence of more than 2 painful ulcers associated with purpura, with no response to treatment | |
| Presence of painful ulcers and lack of response to treatment, located on the trunk, limbs, and penis, associated with purpura |
Diagnosis of calciphylaxis can be made in the presence of the 3 clinical criteria or 2 clinical criteria and pathology.
With regards the imaging tests, plain X-ray imaging shows vascular calcification in very advanced disease states. Modified mammography can assist in the early diagnosis of calciphylaxis. Other techniques such as ultrasound or bone scintigraphy may also be considered. Ultrasonography can reveal echogenic foci with posterior acoustic shadows suggestive of calcification. Bone scintigraphy may be useful for detecting calcium deposits in subcutaneous cell tissue, and so provide an indication of the extension of the lesions, as well as the response to treatment.26
Differential diagnosis of calciphylaxis includes all diseases that may have skin involvement, such as livedo racemosa syndrome, retiform purpura, and skin necrosis/ulcer. Entities to consider include antiphospholipid syndrome, livedoid vasculopathy, disseminated intravascular coagulation, coumarin-induced skin necrosis, type i cryoglobulinemia, thrombophilias, sickle cell anemia, drug-induced anemia (propylthiouracil, cocaine, and levamisole), auricular myxoma, cholesterol, primary hyperoxaluria, Lucio phenomenon, Sneed syndrome, and autoimmune diseases that cause vasculitis (polyarteritis nodosa, Wegener granulomatosis, Churg-Strauss syndrome, microscopic polyangiitis, systemic lupus erythematosus, and rheumatoid arthritis).26,28
Treatment of CCTreatment for CC is complex and should be tailored to the patient (Table 5). There are no controlled clinical trials comparing the different therapeutic alternatives. Knowledge of the efficacy of the different treatments depends on publications of isolated cases or small case series.29
Treatment of Calcinosis Cutis.
| Drugs | Mechanism of Action | Dose | Remarks |
|---|---|---|---|
| Dilthiazem | Reduces calcium uptake by the cells | High doses (2-4mg/kg/d) | Partial or complete response in half the patients. Mainly for dermatomyositis |
| Warfarin | Inhibits carboxylation and reduced MGP levels | Low doses (1mg/d) | It is not known whether it is actually effective given its contradictory mechanism of action |
| Biphosphonates | Inhibit macrophages activated by calcification and inhibit proinflammatory cytokines produced by these cellsDecrease calcium resorption | Oral etidronate: 800mg/dOral alendronate: 70mg/wkIntravenous pamidronate: 90mg/m. | Response to extensive calcifications in dermatomyositis |
| Minocycline | Effect on proteolysis, calcium binding, and antiinflammatory effect | 50-100mg/d | Response in dystrophic calcification of limited systemic sclerosis and mainly on inflammation |
| Ceftriaxone | Effect on proteolysis, calcium binding, and antiinflammatory effect | 2g/d intravenously for 20 days | Response in calcinosis caused by morphea profunda and mainly on inflammation |
| Aluminum hydroxide | Binds phosphate and decreases absorption | 2.24mg/d2.4mg/d1.8mg/d | Response in calcinosis due to dermatomyositis and systemic lupus erythematosus |
| Probenecid | Increases renal phosphate excretion | 1.5mg/d | Response in juvenile dermatomyositis |
For treatments of small calcium deposits, responses have been reported with warfarin, ceftriaxone, and intravenous immunoglobulins.
Surgical excision and destruction with CO2 laser light are also therapeutic options. Larger lesions, on the other hand, may respond to treatment with diltiazem, biphosphonates, probenecid, aluminum hydroxide, and surgical excision or curretage.29–31
DiltiazemDiltiazem is the most widely used treatment for CC. It works by restricting the entry of calcium into cells and macrophages of the affected tissues. Partial or complete response is observed in more than half the patients with CC and the drug is used mainly in CC caused by dermatomyositis. It requires high doses (2-4mg/kg/d) to obtain therapeutic efficacy.29–32
WarfarinA clinical response has been obtained mainly in small calcifications at a dose of 1mg/d. High levels of vitamin K have been detected in patients with CC, and these return to normal after treatment with warfarin.29,30
BiphosphonatesThe mechanism of action of biphosphonates in the treatment of calcinosis depends on their action on macrophages, which are activated in areas with calcium deposits. These drugs therefore inhibit proinflammatory cytokine release from macrophages. In addition, biphosphonates reduce calcium turnover and resorption. Biphosphonates have shown response mainly in dystrophic calcifications of dermatomyositis and systemic sclerosis. Etidronate is used at an oral dose of 800mg/d. Oral alendronate is also used at a dose of 70mg/wk, as is pamidronate, which is infused at a dose of 90mg/wk. The most noteworthy adverse effects of biphosphonates include hypocalcemia, hypophosphatemia, hypomagnesemia, fever, infusion-site reaction, and osteonecrosis of the jaw.29,30,33
MinocyclineMinocycline inhibits collagenolytic enzymes including matrix metalloproteinases. Inhibition of these enzymes is important for reducing inflammation and ulceration. The drug can also chelate calcium. It is used at a dose of 50 to 100mg/d and response has been observed mainly in limited systemic sclerosis.30,34,39
CeftriaxoneCeftriaxone, like tetracyclines, also has an effect on matrix metalloproteinases, calcium binding properties, and antiinflammatory capacity. It has been used with favorable response at intravenous doses of 2g/d for 20 days in the treatment of CC due to morphea profunda.30,39
Aluminum HydroxideCalcified masses contain hydroxyapatite and amorphous calcium phosphate. Aluminum hydroxide can bind to phosphorus and reduce intestinal absorption of phosphorus. It has been used at doses of 2.24g/d, 2.4g/d, and 1.8g/d for the treatment of calcinosis due to dermatomyositis and systemic lupus erythematosos.29,35
ProbenecidProbenecid is an inhibitor of uric acid reuptake in the proximal tubule of the nephron and increases renal phosphate excretion. It has been used with therapeutic response at a dose of 1.5g/d in extensive calcinosis due to juvenile dermatomyosititis.29,30
Topical Sodium ThiosulfateBair et al.36 reported 2 cases of patients with ulcerated dystrophic calcification that remitted after treatment with sodium thiosulfate in powder mixed with zine oxide 1:4 (25% sodium thiosulfate) applied every 12h with occlusion. Of note with these cases is that the topical treatment was applied to the skin that was ulcerated due to CC.
Other TreatmentsColchicine is a drug with antiinflammatory activity, and for this reason it has been used mainly to reduce inflammation.37 Intravenous immunoglobulins at an antiinflammatory dose of 2g/kg have been used with a favorable response in digital calcification in CREST (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome and dermatomyositits.30,38 Intralesional corticosteroids have been used in CC for limited systemic sclerosis.39
Nonmedical TreatmentsExtracorporeal shock-wave lithotripsy has shown a satisfactory response in the treatment of pain in patients with CC. Surgical resection is the mainstay treatment for cases of idiopathic CC such as scrotal calcinosis. Ablative treatment can also be considered for small and digital calcified lesions, where a therapeutic option is treatment with CO2 laser light.30,40,41
In summary, localized lesions are candidates for surgical treatment while generalized lesions require medical treatment. Other surgical indications would be for complications arising from calcium deposits such as pain, recurrent infections, ulceration, and impaired mobility.
Treatment and Prevention of CalciphylaxisTreatment of calciphylaxis is complex and there is no standard treatment protocol. Therefore, prevention becomes important and involves, essentially, interventions for risk factors (Table 6). Calciphylaxis treatment requires, in addition to medical treatment, appropriate care of ulcers, which often need debridement, and pain management. For wound care, the use of hydrocolloid dressings is recommended.42–44
Preventive Measures for Calciphylaxis.
| Serum phosphate levels of 3.5-5.5mg/dLa |
| Serum calcium levels of 8.4-9.5mg/dL |
| Parathyroid hormone levels of 150-300pg/mLb |
| Calcium-phosphorus product less than 55 |
| Precaution with vitamin D and calcium supplements |
| Care with nutritional status to avoid hypoalbuminemia. May require albumin treatment |
| Control of cardiovascular risk factors |
Sodium thiosulfate is the first-line treatment for calciphylaxis not associated with hyperparathyroidism. The mechanism of action of sodium thiosulfate is based on its capacity to dissolve calcium tissue deposits in soluble calcium thiosulfate complexes. In addition, this agent has a vasodilator and antioxidant effect that helps manage pain. The usual dose is 25g (100mL of 25% solution) given over 1h and administered 3 times a week after hemodialysis. Timing is important, given that if we administer the drug during hemodialysis, approximately 50% of the dose is eliminated during the procedure. It is recommended to continue with the drug for at least 2 months after complete ulcer healing. With treatment, the pain usually diminishes rapidly and lesion healing is achieved in weeks or months after starting. This treatment has a good safety profile, and can be used both for calciphylaxis of uremic and nonuremic origin. Noteworthy adverse effects include nausea, vomiting, headache, and rhinorrhea. The most important adverse effect is the development of metabolic acidosis, which can be managed with bicarbonate.45–49 The intralesional route of administration is another possibility which has been reported recently with very good outcomes.50
Other Medical TreatmentsBiphosphonates such as intravenous pamidronate, intravenous ibandronate, and oral etidronate have demonstrated efficacy in the treatment of calciphylaxis. Cinacalcet is a calcimimetic that is used for treating secondary hyperparathyroidism in patients with CKF on dialysis. Cinacalcet could be an alternative to parathyroidectomy in patients with calciphylaxis and hyperparathyroidism.51–54
Hyperbaric ChamberA positive response has been reported in the treatment of calciphylaxis in a hyperbaric chamber. Such an approach may improve healing and decrease the risk of infection due to release of reactive oxygen species. Between 20 and 40 sessions are recommended. The adverse effects are usually mild and the safety profile of the treatment is good.55
ConclusionsDiseases arising from disturbed calcium and phosphorus metabolism that affect the skin can be classed as CC or calciphylaxis. The most affected group of patients are those with renal disease. In the case of CC, the laboratory tests can provide an indication of the etiology while imaging tests can reveal the extension of the disease. We decide whether or not to treat and the type of treatment (medical or surgical) according to degree of clinical involvement. Calciphylaxis is a serious disease with a high mortality that occurs mainly in patients with terminal CKF on hemodialysis. It presents some clinical characteristics that can guide diagnosis. The mainstay treatment is intravenous sodium thiosulfate. This treatment can induce remission of skin ulcers secondary to calciphylaxis with rapid pain relief.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Jiménez-Gallo D, Ossorio-García L, Linares-Barrios M. Calcinosis cutis y calcifilaxis. Actas Dermosifiliogr. 2015;106:785–794.