Chronic spontaneous urticaria (CSU) is defined by the appearance of a >6-week history of wheals and/or angioedema without an identifiable triggering agent and significantly impacting the patients’ quality of life.1
Omalizumab is associated with high efficacy in the treatment of CSU.1 Furthermore, dose escalation, as recommended by clinical practice guidelines, can benefit most patients who exhibit a lack of response or partial response. On the other hand, treatment with ciclosporin (CsA) – recommended as a third-line therapy –has also been associated with high response rates, particularly in cases of type IIb autoimmunity.2 Therefore, non-responders to both omalizumab and CsA represent a small percentage of challenging-to-treat patients. However, there are no specific recommendations for further treatments in these patients, as alternative treatment options have limited low-quality evidence.1
We report our experience with 7 cases of CSU refractory to antihistamines, omalizumab and CsA treated with azathioprine across 3 dermatology departments of tertiary referral centers. The cohort included a total of 5 women and 2 men, with a median age of 69 years (range, 17–85). Two patients (29%) exhibited concurrent chronic inducible urticaria (symptomatic dermographism and delayed pressure urticaria) and 5 (71%), angioedema. Additionally, 2 patients (29%) exhibited autoimmune thyroid disease, and 1 patient (14%), type 1 diabetes mellitus. Baseline blood test revealed the presence of anti-thyroid peroxidase antibodies in 3 patients, while median basal values of immunoglobulin E and D-dimer were 45kU/L (2–1043) and 485ng/mL (170–766), respectively. The median time of omalizumab and CsA treatment prior to azathioprine initiation were 11 (3–43) and 23 months (4–31), respectively.
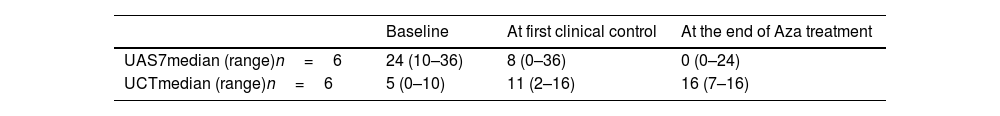
The median dose of azathioprine was 1.2mg/kg/day (0.5–2.3). Omalizumab was maintained in 4 (57%) patients while on azathioprine. Median values of the Urticaria Activity Score 7 (UAS7) and Urticaria Control Test (UCT) questionnaires – available for 6 out of 7 patients – are shown in Table 1.
Results of UAS7 and UCT questionnaires prior, at the first control (after a median of 2 (1–4.2) months after starting azathioprine), and at the end of azathioprine therapy (after 10 (1.8–11) months of therapy).
| Baseline | At first clinical control | At the end of Aza treatment | |
|---|---|---|---|
| UAS7median (range)n=6 | 24 (10–36) | 8 (0–36) | 0 (0–24) |
| UCTmedian (range)n=6 | 5 (0–10) | 11 (2–16) | 16 (7–16) |
UAS7: Urticaria Activity Score 7; UCT: Urticaria Control Test; Aza: azathioprine.
A complete response (UAS7=0 and UCT=16) was achieved in 4 (57%) patients after 3.2 months (1–7.2), 2 of them along with omalizumab. Two of these patients are still on azathioprine, and the remaining 2 discontinued treatment due to symptom resolution with no relapse after discontinuation and no need for further treatment. The remaining 3 patients showed no response, one of them receiving a reduced dose of azathioprine of 0.5mg/kg/day due to a thiopurine methyltransferase activity of 11.6U/mL. Another non-responsive patient received the treatment for only 1.8 months due to hepatotoxicity, which resolved after treatment discontinuation. Two out of the 3 patients who remained unresponsive to azathioprine had an inducible urticaria.
Although failed omalizumab+CsA therapy is a rare finding, some patients still require further therapeutic options. Furthermore, CsA is not recommend for long-term use due to the high risk of renal damage.2 Azathioprine – a purine synthesis inhibitor – induces apoptosis of T lymphocytes.3 Azathioprine has proven effective in CSU cases refractory to antihistamines4,5 or CsA.3 In cases refractory to antihistamines, treatment with azathioprine has proven non-inferior to CsA, with similar outcomes achieved at 2 months and maximum response observed around 3 months of treatment5 as seen in patients included in our study. The non-responsive case observed in our series could be attributed to insufficient dosing due to TPMT activity in one patient, or limited treatment duration because of adverse event in another.
To this date, no cases of azathioprine after omalizumab and CsA treatment have been reported. Based on our results, azathioprine could represent an option for these very difficult-to-treat cases of CSU. While azathioprine was generally well tolerated, one case of hepatitis was identified, highlighting the necessity for close monitoring during treatment.
Patient consentThe patients in this manuscript have given written informed consent to publication of their case details.
Ethical approvalData were retrieved from the medical records and from a prospective registry started in 2009, which received the approval of our local Ethics Committee (16/049 (OBS)), enrolling patients with CSU.
FundingNone declared.
Conflicts of interestLMA and RM declared no conflicts of interest whatsoever. VES has been medical advisor and speaker for Abbvie, Lilly, LEO Pharma, Novartis, Almirall and Sanofi Genzyme. AAA declared to have received speaker fees by Novartis. ES declared to have received honoraria for speaking engagements, for his involvement in advisory boards, and advisory roles for the following pharmaceutical companies: Lilly, Amgen, Leo Pharma, Novartis, Pfizer, Sanofi, Abbvie, and Pierre Fabre. JS declared to have received speaker fees. Moreover, the has been involved in clinical trials sponsored by Abbvie, Lilly, LEO Pharma, Novartis and Sanofi Genzyme.