It has been suggested that patients who have had a melanoma may develop increased immunity against certain antigens expressed by tumor-associated melanocytes. Thus our objective was to review the records of patients with successive primary melanomas to ascertain whether the pattern of regression might indicate the presence of an immunization effect arising from the first melanoma.
Material and methodsA review of all the cases recorded in the melanoma database of our dermatology department between 2000 and 2012 identified 19 patients who had multiple asynchronous melanomas (2.56% of all the cases recorded). We studied the presence or absence of regression in these melanomas and other clinical and histological characteristics.
ResultsThe presence of regression was significantly higher in successive melanomas than in the first tumors identified (42.10% vs 21.05%, P=.018). Regression of at least 1 melanoma was observed in 42.10% of the patients studied and regression of 2 melanomas was observed in 21.05%. In no case was regression observed in the first melanoma and not in the second; however, in 21.05% of the patients there was evidence of regression in the second tumor and none in the first.
ConclusionsOur findings suggest the possibility that the first melanoma produces an immunization effect in some patients who develop multiple asynchronous melanomas.
Se ha especulado que en los pacientes que han padecido un melanoma puede existir un aumento de la inmunidad frente a determinados antígenos que expresan los melanocitos tumorales. De este modo, nos planteamos revisar la regresión en pacientes con melanomas primarios sucesivos, que podría constituir un reflejo de ese efecto inmunizante que ejercería el primer melanoma.
Material y métodosUtilizando la base de datos de melanomas de nuestro Servicio de Dermatología, en el período 2000-2012 se han identificado un total de 19 pacientes con melanomas múltiples no simultáneos (2.56% del total). En esos melanomas se ha estudiado la presencia o ausencia de regresión, junto a otras características clínicas e histológicas de los mismos.
ResultadosLa presencia de regresión en los últimos melanomas extirpados fue significativamente superior a la observada en los primeros (42.10% frente a un 21.05% respectivamente; p=0.018). Un 42.10% de los pacientes experimentaron regresión en al menos uno de sus melanomas. Se evidenció presencia de regresión en los dos melanomas pertenecientes al mismo paciente en un 21.05%. No hubo ningún caso con regresión en el primer melanoma y ausencia de la misma en el segundo, mientras que se apreció regresión en el segundo melanoma y ausencia de la misma en el primero en un 21.05% de los pacientes.
ConclusionesEstos hallazgos sugerirían la posibilidad de un efecto inmunizante por el primer melanoma en algunos pacientes con melanoma múltiples no simultáneos.
Melanoma is among the tumors that are most able to stimulate the immune response. Regression of melanoma is 6 times more frequent than in other malignant neoplasms,1 and even though complete regression of melanoma is exceptional, partial regression is common, occurring in 10% to 35% of cases.2
Several mechanisms may be involved in the induction of regression, although an immune mechanism seems to be the most consistently associated with the process.3,4
Therefore, it has been proposed that patients who have had melanoma may have increased immunity to specific antigens expressed by tumor-associated melanocytes.5–8
With the aim of investigating a possible immunization effect after the first melanoma, we studied regression of the first and successive melanomas in a series of patients with more than 1 primary cutaneous melanoma. We expected that regression rates of the most recently removed melanomas would be superior to those of older melanomas.
Material and MethodsAfter reviewing the melanoma database of our dermatology department, we selected all patients diagnosed consecutively with multiple melanomas between 2000 and 2012. We then performed a retrospective study by recording several clinical and pathology findings obtained from medical histories and from hematoxylin-eosin–stained paraffin sections.
Clinical DataWe recorded age at removal of the first melanoma, sex, site of the first melanoma, interval between excision of the first melanoma and the second, and patient outcome.
Histologic DataFor both the first melanoma and the second melanoma, we recorded histologic type, Breslow thickness, absence or presence of regression and percentage of regression.
Regression was assessed based on horizontal spread according to the guidelines proposed by the Autonomous Community of Valencia.9 The results were expressed as absence or presence of regression; if regression was present, it was classified according to whether it was observed in ≤50% or >50% of the tumor area. Histologic regression was defined as the replacement of tumor cells by a fibrous stroma, with no or very few tumor-associated melanocytes, but with melanophages, lymphocytes, and newly formed vessels, as suggested by other authors.2,10 Inflammation predominated in the early or active stages, whereas fibrosis predominated later in the process.
Statistical AnalysisQuantitative variables were expressed as mean (SD) and range. The means of 2 independent groups were compared using the t test; if the variables did not fulfill the criteria for normality, the Mann-Whitney test was used. The Shapiro-Wilk test was used to evaluate the normality of the distribution of the variables included in the analysis. Qualitative variables were expressed as absolute values and percentages and compared using the χ2 test or the Fisher exact test when the expected value of 1 or more cells was<5.
A P value≤.05 was considered significant. Data were analyzed using SPSS, version 18.0.
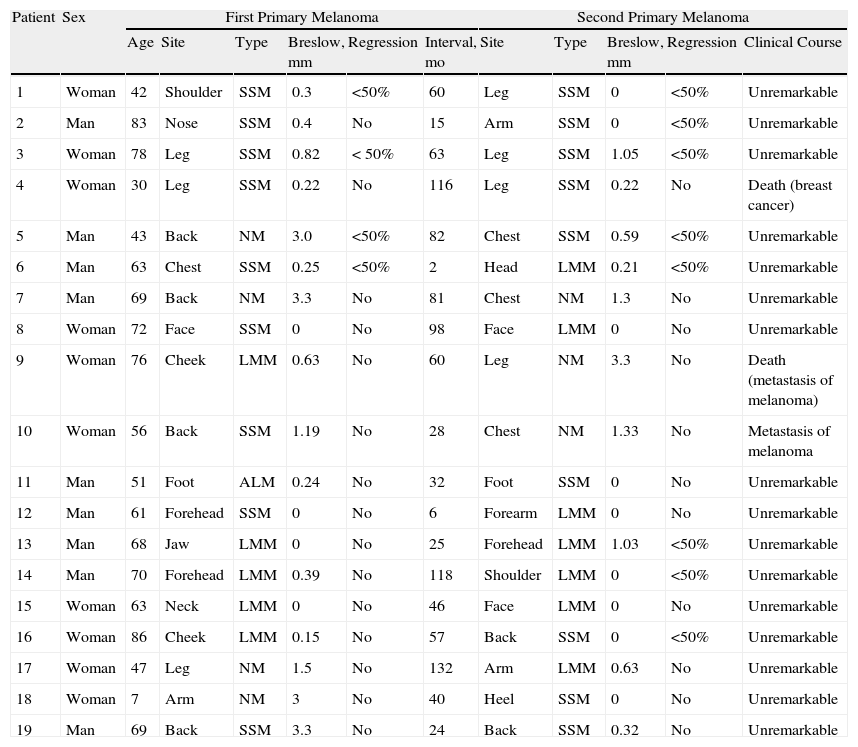
ResultsDescriptive Analysis (Table 1)A review of the melanoma database of our dermatology department (741 patients during 2000-2012) revealed 19 patients with multiple melanomas (2.56% of the series). No patient had had more than 2 melanomas removed, and the excisions were separated by an interval of at least 2 months in all cases. (Table 1)
Summary of the Main Clinical and Histological Parameters in Patients With Successive Melanomas Analyzed in the Present Series.
| Patient | Sex | First Primary Melanoma | Second Primary Melanoma | |||||||||
| Age | Site | Type | Breslow, mm | Regression | Interval, mo | Site | Type | Breslow, mm | Regression | Clinical Course | ||
| 1 | Woman | 42 | Shoulder | SSM | 0.3 | <50% | 60 | Leg | SSM | 0 | <50% | Unremarkable |
| 2 | Man | 83 | Nose | SSM | 0.4 | No | 15 | Arm | SSM | 0 | <50% | Unremarkable |
| 3 | Woman | 78 | Leg | SSM | 0.82 | < 50% | 63 | Leg | SSM | 1.05 | <50% | Unremarkable |
| 4 | Woman | 30 | Leg | SSM | 0.22 | No | 116 | Leg | SSM | 0.22 | No | Death (breast cancer) |
| 5 | Man | 43 | Back | NM | 3.0 | <50% | 82 | Chest | SSM | 0.59 | <50% | Unremarkable |
| 6 | Man | 63 | Chest | SSM | 0.25 | <50% | 2 | Head | LMM | 0.21 | <50% | Unremarkable |
| 7 | Man | 69 | Back | NM | 3.3 | No | 81 | Chest | NM | 1.3 | No | Unremarkable |
| 8 | Woman | 72 | Face | SSM | 0 | No | 98 | Face | LMM | 0 | No | Unremarkable |
| 9 | Woman | 76 | Cheek | LMM | 0.63 | No | 60 | Leg | NM | 3.3 | No | Death (metastasis of melanoma) |
| 10 | Woman | 56 | Back | SSM | 1.19 | No | 28 | Chest | NM | 1.33 | No | Metastasis of melanoma |
| 11 | Man | 51 | Foot | ALM | 0.24 | No | 32 | Foot | SSM | 0 | No | Unremarkable |
| 12 | Man | 61 | Forehead | SSM | 0 | No | 6 | Forearm | LMM | 0 | No | Unremarkable |
| 13 | Man | 68 | Jaw | LMM | 0 | No | 25 | Forehead | LMM | 1.03 | <50% | Unremarkable |
| 14 | Man | 70 | Forehead | LMM | 0.39 | No | 118 | Shoulder | LMM | 0 | <50% | Unremarkable |
| 15 | Woman | 63 | Neck | LMM | 0 | No | 46 | Face | LMM | 0 | No | Unremarkable |
| 16 | Woman | 86 | Cheek | LMM | 0.15 | No | 57 | Back | SSM | 0 | <50% | Unremarkable |
| 17 | Woman | 47 | Leg | NM | 1.5 | No | 132 | Arm | LMM | 0.63 | No | Unremarkable |
| 18 | Woman | 7 | Arm | NM | 3 | No | 40 | Heel | SSM | 0 | No | Unremarkable |
| 19 | Man | 69 | Back | SSM | 3.3 | No | 24 | Back | SSM | 0.32 | No | Unremarkable |
Abbreviations: ALM, acral lentiginous melanoma; LMM, lentigo maligna melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma.
Distribution by sex was almost even (10 females and 9 males). Mean age at diagnosis of the first melanoma was 59.6 (19.5) years (range, 7-86 years). The first melanoma was found mostly on the head (8 cases, 42.10%), followed by the back (4 cases, 21.05%) and lower limbs (4 cases, 21.05%). Histopathology revealed 9 superficial spreading melanomas (47.36%), 5 lentigo maligna melanomas (26.31%), 4 nodular melanomas (21.05%), and 1 acral lentiginous melanoma (5.26%). The mean Breslow thickness was 0.99 (1.21) mm (range, 0-3.3mm). Regression was recorded in 4 cases (21.05%) and was <50% in all 4. Regression was found in 3 superficial spreading melanomas (33.3%) and in 1 nodular melanoma (25%). The mean interval between excision of the first and second melanomas was 57.10 (39.95) months (range, 2-132 months).
The second melanomas were found mostly on the lower limbs (6 cases, 31.57%), followed by the upper limbs (4 cases, 21.05%) and head (4 cases, 21.05%). Histopathology revealed 9 superficial spreading melanomas (47.36%), 7 lentigo maligna melanomas (36.84%), and 3 nodular melanomas (15.78%). Nine patients (47.36%) developed the same type of melanoma. The mean Breslow thickness in the second melanoma was 0.52 (0.82) mm (range, 0-3.3mm).
Regression of the second melanoma was identified in 8 cases (42.10%), and, again, was <50% in all of them. Regression was observed in 5 superficial spreading melanomas (55.5%) and in 3 lentigo maligna melanomas (42.80%). It is noteworthy that even though 26.3% of the first melanomas were lentigo maligna melanoma, regression was not observed in any of them. In contrast, when the second melanoma was also lentigo maligna melanoma, as many as 42.8% regressed. Therefore, regression was considerably more frequent in cases where the second melanoma evolved from a lentigo maligna. In the case of superficial spreading melanoma, regression was more frequent in the second tumor (44.4% vs 55.5%).
Regression in at least 1 melanoma was observed in 8 patients (42.10%). Regression was observed in 2 melanomas in the same patient in 4 cases (21.05%); absence of regression in the 2 melanomas was observed in 11 patients (57.89%). Regression in the second melanoma was never greater than in the first. There were no cases of regression in the first melanoma and absence of regression in the second. In contrast, regression was observed in the second melanoma but not in the first in 4 cases (21.05%), thus potentially indicating the presence of an immunization effect.
As for the effect of the interval between excisions and the development of regression in the second melanoma, the mean interval was 51.75 (34.56) months in cases where both melanomas had regressed. When regression was only observed in the second melanoma, the mean interval was 53.75 (46.48) months; when regression was not observed in either melanoma, the interval was 61.18 (40.77) months.
The disease did not progress in any of the male patients, although 2 of the female patients (20%) developed metastasis; regression was not observed in the primary melanomas in either of the 2 patients. Furthermore, 1 of the patients with metastasis had 2 melanomas with a Breslow thickness of 1.19mm and 1.33mm. A third patient died of breast cancer, although no regression of her melanomas was observed.
Statistical AnalysisRegression in the second melanoma was significantly more frequent than in the first (42.10% vs 21.05%, respectively; P=.018).
Analysis of the associations between the remaining variables studied (sex, site, histologic type, age, Breslow thickness, and interval) and the presence of regression (first and second melanoma) did not reveal statistically significant differences.
DiscussionThe incidence of multiple melanomas in the present series was 2.6% (95% CI, 1.4%-3.8%), which is consistent with the findings of other authors, who established incidence at 1%-4% of all patients with melanoma.5
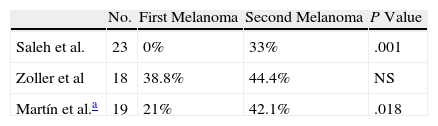
Very few studies have attempted to assess whether antitumoral immunity against melanoma-associated antigens is greater in successive melanomas in patients with several primary melanomas. The results obtained to date seem contradictory (Table 2).5–7
Percentages of First and Successive Primary Melanomas With Regression in Case Series Reported in the Literature.
| No. | First Melanoma | Second Melanoma | P Value | |
| Saleh et al. | 23 | 0% | 33% | .001 |
| Zoller et al | 18 | 38.8% | 44.4% | NS |
| Martín et al.a | 19 | 21% | 42.1% | .018 |
Abbreviations: NS, nonsignificant.
There has been speculation that recognition of melanocytic differentiation antigens by the immune system could lead to increased destruction of tumors in successive melanomas, provided that they share the same antigens that induce the immune response, which would manifest as histologic regression of the last primary tumor.6
Various types of antigen are expressed in melanomas and can be recognized by cytotoxic T lymphocytes.11–13 The most frequently detected group of antigens in patients with melanoma—and the most immunogenic—is that comprising melanocytic differentiation antigens. This category includes the melanoma antigens Melan-A/MART-1 (the most common), gp100, and tyrosinase.14 The genes coding these antigens are expressed not only in melanomas, but also in normal melanocytes.8,15 In fact, in patients with melanoma, specific cytotoxic T lymphocytes against this type of antigen can be isolated both in peripheral blood and in lymph nodes and tumor tissue. Similarly, in patients with complete regression of multiple melanocytic nevi after developing melanoma, cytotoxic lymphocytes against gp100 and MART-1 have been detected both in peripheral blood and in regressing nevi, thus suggesting rupture of immune tolerance against self-antigens.8
Saleh et al.6 studied regression in 23 patients with multiple primary melanoma and in a control group of patients with a single melanoma. They found no differences between the percentages of histologic regression in the first melanomas in the group of patients with multiple melanomas and the control group (a single melanoma). Surprisingly, in the group with multiple melanomas, a significant increase in regression was observed in the last melanomas removed when they were compared with the oldest melanomas and with the single melanomas in the control group. The same authors found significantly reduced expression of MART-1 in the last primary melanoma in the group of patients with various melanomas and in the other group of metastatic melanomas in patients whose primary tumor had completely regressed. They also observed a correlation between the presence of MART-1–specific cytotoxic lymphocytes in peripheral blood and loss of the MART-1 tumor antigen. This finding was attributed to an immunization effect against regression of multiple asynchronous primary melanomas.
Zoller et al.5 also studied regression in 18 patients with 2 primary melanomas that were removed with an interval of at least 1 month between them. In contrast with the previous study, although the authors identified regression in 38.8% of first melanomas and in 44.4% of successive melanomas, the differences were not statistically significant, thus precluding the hypothesis of an immunization effect generated by the primary melanoma.
Using molecular profiling, Orlow et al.7 showed that none of the cases of multiple primary melanomas in the same patient were clonal in origin. In our study, 52.68% of the patients also developed different histologic types of melanoma. These findings suggest that, in addition to antigens associated with common tumors in most melanomas and also in melanocytic nevi, such as MART-1,8 other tumor-associated antigens are unique for each type of primary melanoma and might not contribute to an immunization effect.
Regression was<50% in all the cases we report; therefore, although an immune response was triggered, it did not completely destroy the tumor. Given that melanoma is among tumors that have a high number of somatic mutations and that partial regression—unlike complete regression—is relatively common, the most frequent antigens may be those with the greatest capacity to induce an immune response. It is also possible that cell clones are generated with the self-antigens of a specific melanoma that would not activate the immune response or that would develop mechanisms of escape from the pressure they are subjected to by the immune system. Therefore, in some cases, regression of the second melanoma could be independent of regression of the first melanoma.
We found the percentage of regressed second melanomas to be double that of first melanomas; the differences were statistically significant.
There has been speculation that regression develops mainly in the initial stages of pathogenesis, thus pointing to early activation of the immune response, since this is much more frequent in the radial growth phase and in thin melanomas.2 In our study, the mean Breslow thickness in second melanomas was 0.52(0.82) mm (range, 0-3.3mm), which was considerably lower than that of the first ones. The explanation for this difference would be that, since these patients are closely monitored because of their previous melanoma, the second melanomas were removed early, as reflected by the fact that most were melanoma in situ (47.36% vs 21.05% in the first melanomas).
The statistical analysis showed that the interval between excisions would not account for the differences found in the cases we report.
The biologic significance of regression of melanoma is much debated in the literature. More recent studies tend to consider regression to be a protective mechanism,2,16 that is, an attempt by the body to destroy the tumor.
Regression of a melanoma starts with an accompanying inflammatory infiltrate, which indicates that a host immune response to a tumor has taken place. Furthermore, the presence of abundant tumor-infiltrating lymphocytes in the vertical growth phase of primary melanomas has been consistently associated with a reduction in the incidence of metastatic disease and greater survival.17 One of the most notable advances in the treatment of melanoma is based on the development of therapies that boost the immune response to the tumor, such as the monoclonal antibody ipilimumab, which blocks cytotoxic T lymphocyte antigen 4 (CTLA-4), or the human antibody that acts against the programmed cell death 1 (PD1) molecule. Both CTLA-4 and binding of PD1 to its ligand (PD-L1) limit the immune response to melanoma.18
Patients who spontaneously develop an immune response may obtain a more marked benefit from the use of these immunomodulatory antibodies.
As for prognosis, 10.52% of the patients we report developed metastasis, although none of their melanomas regressed. In contrast, none of the patients whose melanomas regressed developed metastasis.
ConclusionsThe results of this series point to an increase in the incidence of regression in successive melanomas with respect to primary melanomas, thus reflecting a potential immunization effect induced by specific melanomas.
The fact that metastasis was observed exclusively in patients whose melanomas did not regress would lend support to the idea that regression could constitute a protective mechanism.
Ethical DisclosuresProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martín JM, Pinazo I, Mateo JF, Escandell I, Jordá E, Monteagudo C. Evaluación de la regresión en melanomas primarios sucesivos. Actas Dermosifiliogr. 2014;105:768–773.