In the past 10 years, bullous pemphigoid has been associated with other comorbidities and neurologic and psychiatric conditions in particular. Case series, small case-control studies, and large population-based studies in different Asian populations, mainland Europe, and the United Kingdom have confirmed this association. However, no data are available for the Spanish population.
Material and methodsThis was an observational, retrospective, case-control study with 1:2 matching. Fifty-four patients with bullous pemphigoid were selected. We compared the percentage of patients in each group with concurrent neurologic conditions, ischemic heart disease, diabetes, chronic obstructive pulmonary disease, and solid tumors using univariate logistic regression. An association model was constructed with conditional multiple logistic regression.
ResultsThe case group had a significantly higher percentage of patients with cerebrovascular accident and/or transient ischemic attack (odds ratio [OR], 3.06; 95% CI, 1.19-7.87), dementia (OR, 5.52; 95% CI, 2.19-13.93), and Parkinson disease (OR, 5; 95% CI, 1.57-15.94). A significantly higher percentage of cases had neurologic conditions (OR, 6.34; 95% CI, 2.89-13.91). Dementia and Parkinson disease were independently associated with bullous pemphigoid in the multivariate analysis.
ConclusionsPatients with bullous pemphigoid have a higher frequency of neurologic conditions.
En la última década se ha relacionado el penfigoide ampolloso (PA) con la presencia previa de comorbilidades, sobre todo enfermedades neurológicas y psiquiátricas. Disponemos en la bibliografía de series de casos, pequeños estudios de casos y controles y trabajos extensos con bases de datos poblacionales en distintos países asiáticos, de Europa continental y del Reino Unido que han confirmado esta asociación. Sin embargo, no existen datos en la población española.
Material y métodosEstudio observacional retrospectivo de casos y controles con matching 1:2. Se seleccionaron 54 pacientes con PA. Se compararon proporciones entre grupos para comorbilidades neurológicas, cardiopatía isquémica, diabetes, enfermedad pulmonar obstructiva crónica (EPOC) y neoplasias sólidas mediante regresiones logísticas univariantes. Se creó un modelo asociativo mediante regresión logística múltiple condicional.
ResultadosEn el grupo de casos aparece una proporción significativamente mayor de accidente cerebrovascular y/o accidente isquémico transitorio (ACVA y/o AIT) (odds ratio [OR] 3,06; intervalo de confianza 95% [IC 95%] 1,19-7,87], demencia (OR: 5,52; IC 95%: 2,19-13,93) y enfermedad de Parkinson (OR: 5; IC 95%: 1,57-15,94). Tomando los diagnósticos neurológicos en conjunto se advirtió una proporción significativamente mayor en casos (OR: 6,34; IC 95%: 2,89-13,91). Demencia y enfermedad de Parkinson se asociaron de forma independiente al PA en el análisis multivariante.
ConclusionesLos pacientes con PA de nuestra población presentan mayor frecuencia de ciertas comorbilidades neurológicas.
Bullous pemphigoid (BP) is a subepidermal blistering disease that typically affects older individuals,1–4 with risk increasing with age.5 Patients frequently develop tense blisters on a pruritic, urticarial base. BP is an immune-mediated disease characterized by the production of immunoglobulin G autoantibodies directed against hemidesmosomal antigens at the dermal-epidermal junction, giving rise to C3 deposition and subsequent formation of blisters.
The hemidesmosomal antigens are BP180 (BPAG2 or collagen xvii), a transmembrane protein with a long extracellular domain, and BP230 (BPAG1), an intracellular protein belonging to the plakin family. Several isoforms of BPAG1 have been described: epithelial BPAGI or BPAGI-e in the skin, BPAG1-b in the striated muscle, and BPAG1-a (which is very similar to BPAGI-e) in the nervous system.6
In recent years, a number of studies have described an epidemiological association between BP and several neurological conditions, such as cerebrovascular accident (CVA), certain forms of dementia, and Parkinson disease.7–9 While the pathogenic mechanism underlying this association is unknown, one group of authors suggested that it might involve cross-reactivity between the epithelial and neuronal isoforms of BPAG1 following the neurological event.10 This would expose the neuronal isoform to the immune system, triggering an autoantibody response against the epithelial isoform and resulting in BP. This mechanism was postulated on demonstrating experimentally that sera from patients with BP and a neurological disorder recognized BP antigen 1 in the skin and brain of mice,11 offering a possible explanation for how bullous pemphigoid might develop in patients with a preexisting neurological condition.
We have conducted what is, to our knowledge, the first study of the association between BP and neurological disease in Spanish patients.
Patients and MethodsWe designed a retrospective, observational case-control study in which we selected all incident cases of BP recorded between January 2002 and February 2012. The cases were obtained from the electronic medical record database at the dermatology department of Hospital Universitario Reina Sofía in Córdoba, Spain. Cases were identified as patients with a clinical diagnosis of BP and compatible histologic and direct immunofluorescence findings.
For each case, we recorded age at the time of diagnosis, sex, BP severity, and all conditions diagnosed up to 3 months before the diagnosis of BP. BP severity was classified as generalized (affecting 2 or more anatomic locations) or localized. The comorbidities recorded were diabetes mellitus, ischemic heart disease, chronic obstructive pulmonary disease, a history of CVA and/or transient ischemic attack, dementia, Parkinson disease, psychiatric disease, solid tumors, and presence of a neurological condition (dementia, CVA, transient ischemic attack, Parkinson disease). The psychiatric disorders observed were anxiety disorder, depression, schizophrenia, and bipolar disorder. To be included, the diseases had to have been diagnosed or confirmed by a primary care physician or a specialist.
Two age- and sex-matched controls were selected for each case from the same database. To qualify as a control, the patient had to have undergone a skin biopsy in the same year as the case for a reason other than a suspected blistering disease. The controls were then matched to cases in groups defined by sex and age by simple random sampling using a random number generator. The records of the controls were screened for the same conditions as those recorded in the cases.
We performed a descriptive analysis of the study variables, using frequencies and percentages for qualitative variables and means and SD for quantitative variables. Differences between means were evaluated using the t test for independent data following testing for normality of distribution where appropriate using the Shapiro-Wilk test. To compare proportions within the group of cases, we used the χ2 test or the Fisher exact test for expected values of less than 5.
Crude odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using univariate logistic regression analysis for both groups to compare the odds of developing BP for each comorbid condition. The resulting ORs were also adjusted for age and sex using the same method. An association model was constructed using BP diagnosis as the dependent variable. The comorbidities (excluding the group of neurologic conditions) were used as independent variables. The model used was a conditional multiple logistic regression model with a 1:2 matching ratio for cases and controls. Variables with a P value of 0.15 or more were successively eliminated (backward stepwise regression) using the Wald statistic. The likelihood ratio test was used to compare the reduced model with the model containing all the variables.
Statistical significance was set at a P value of less than .05 for all tests. All statistical analyses were performed using IBM SPSS Statistics package, version 19.0.0.1.
ResultsData were collected for 56 patients with BP: 22 men (39.3%) and 34 women (60.7%). The mean (SD) age at diagnosis was 80.79 (7.11) years for men and 77.90 (13.07) years for women (P=.354). Forty-eight cases (85.71%) were aged over 70 years. Just 6 patients (10.7%) had localized disease. The remainder (89.3%) had lesions in more than 2 locations.
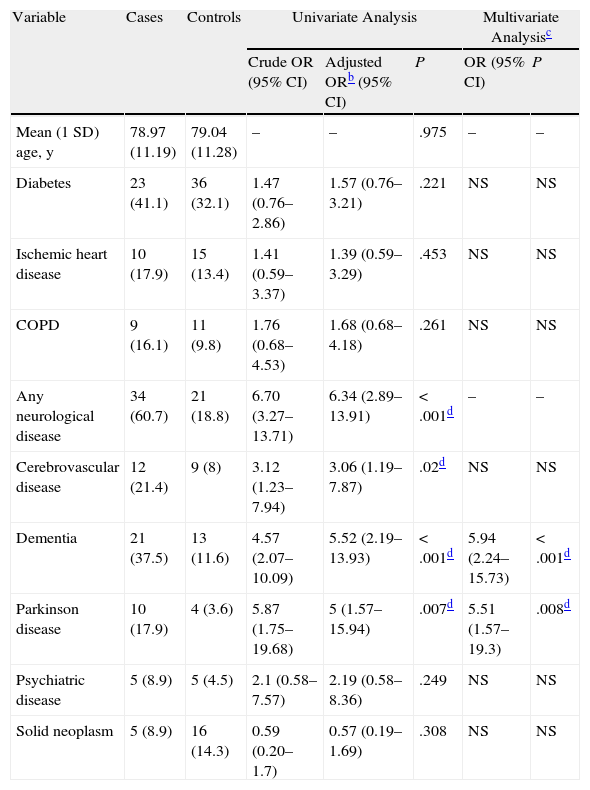
Table 1 shows the results of the univariate and multivariate analyses. The proportion of neurological conditions was almost 7 times higher in cases than in controls (OR, 6.70; 95% CI, 3.27-13.71). Specifically, cases had a significantly increased history of cerebrovascular disease (OR,3,12; 95% CI, 1.23-7.94), dementia (OR,4.57; 95% CI, 2.07-10.09), and Parkinson disease (OR,5,87; 95% CI, 1.75-19.68). No significant differences were found for the other variables. The results remained unchanged on adjusting for age and sex. In the multivariate analysis, dementia (OR, 5.94; 95% CI, 2.24-15.73) and Parkinson disease (OR, 5.51: 95% CI, 1.57-19.3) were both independently associated with BP (Table 1).
Univariate and Multivariate Analysis of Study Variables in Cases and Controls.a
| Variable | Cases | Controls | Univariate Analysis | Multivariate Analysisc | |||
| Crude OR (95% CI) | Adjusted ORb (95% CI) | P | OR (95% CI) | P | |||
| Mean (1 SD) age, y | 78.97 (11.19) | 79.04 (11.28) | – | – | .975 | – | – |
| Diabetes | 23 (41.1) | 36 (32.1) | 1.47 (0.76–2.86) | 1.57 (0.76–3.21) | .221 | NS | NS |
| Ischemic heart disease | 10 (17.9) | 15 (13.4) | 1.41 (0.59–3.37) | 1.39 (0.59–3.29) | .453 | NS | NS |
| COPD | 9 (16.1) | 11 (9.8) | 1.76 (0.68–4.53) | 1.68 (0.68–4.18) | .261 | NS | NS |
| Any neurological disease | 34 (60.7) | 21 (18.8) | 6.70 (3.27–13.71) | 6.34 (2.89–13.91) | <.001d | – | – |
| Cerebrovascular disease | 12 (21.4) | 9 (8) | 3.12 (1.23–7.94) | 3.06 (1.19–7.87) | .02d | NS | NS |
| Dementia | 21 (37.5) | 13 (11.6) | 4.57 (2.07–10.09) | 5.52 (2.19–13.93) | <.001d | 5.94 (2.24–15.73) | <.001d |
| Parkinson disease | 10 (17.9) | 4 (3.6) | 5.87 (1.75–19.68) | 5 (1.57–15.94) | .007d | 5.51 (1.57–19.3) | .008d |
| Psychiatric disease | 5 (8.9) | 5 (4.5) | 2.1 (0.58–7.57) | 2.19 (0.58–8.36) | .249 | NS | NS |
| Solid neoplasm | 5 (8.9) | 16 (14.3) | 0.59 (0.20–1.7) | 0.57 (0.19–1.69) | .308 | NS | NS |
Abbreviations: COPD, chronic obstructive pulmonary disease; NS, not significant.
The prevalence of neurological disease in the general Spanish population is estimated at between 13% and 16%.12 The prevalence of dementia is 10.9% in Spanish individuals aged over 70 years,13 while that of cerebrovascular disease in those aged over 65 years is estimated at between 4.9%14 and 7.5%.15
A range of neurological conditions have been described in patients with BP, including multiple sclerosis, paralysis due to ischemia, multiple system atrophy, and amyotrophic lateral sclerosis.16–20
In recent years, several studies have reported an increased prevalence of certain neurodegenerative diseases in patients with BP. In 2005, for instance, Stinco et al.21compared the prevalence of BP in hospitalized patients with either multiple sclerosis or Parkinson disease and patients who had been hospitalized for trauma during the same period. BP was significantly more common in the first group of patients. In another uncontrolled study that investigated the prevalence of neurological disorders in 341 patients with BP, 123 patients (36%) were found to have a history of at least 1 neurological condition. The most common condition was dementia (20%) (primarily Alzheimer disease and vascular dementia), followed by ictus (15%) and Parkinson disease (9%). The prevalence of multiple sclerosis, epilepsia, amyotrophic lateral sclerosis, and syringomyelia was less than 5%.
The above observations were subsequently confirmed in several controlled studies. In one of these, neurological disease was detected in 42 patients with BP; this corresponded to a prevalence of 46%, which was significantly higher than the rate seen in the control group.9 In the same study, patients with BP had a 6-fold higher odds of having dementia (prevalence, 13%) and an 8-fold higher odds of having cerebrovascular disease (prevalence, 30%) compared with controls. In another case-control study, the proportion of patients with BP in association with a neurological condition (42.7%) was similar to rates reported in other studies.22 None of the other differences detected between cases and controls were statistically significant. Bastuji-Garin et al.,23 in turn, observed that, compared with controls, patients with BP were more likely to be bedridden (38.5%) or to have cognitive impairment (42.7%), ictus (25.9%), Parkinson disease (14.3%), or bipolar disorder (4%). All of the conditions except ictus were independent risk factors in the multivariate analysis.
In a recent case-control study, the proportion of BP patients with neurological disease (16%) was considerably lower than previously reported.24 Despite this lower proportion, however, the differences with controls were still significant. Unlike previous studies, the authors also observed a higher prevalence of neoplasms in cases than in controls. The different results may be attributable to the nature of the population studied.24
Two case-control studies using large population-based databases in Asia7 and the United Kingdom8 have confirmed the results of earlier studies, adding more weight to the evidence available to date.
A review of the literature suggests that our study is the first to explore the association between BP and comorbidities in the Spanish population. Our findings show that certain neurological conditions are more common in patients with BP, which again is consistent with results published to date.
Our study, however, has some limitations, including the very small sample size, which probably prevented us from detecting more differences between cases and controls, such as for example, a greater prevalence of psychiatric disorders, which larger studies have been found to be significantly more common in patients with BP. Our findings may also have been affected by information bias, as the controls were selected from a skin biopsy database. Patients may be referred for a skin biopsy for various reasons, and it is therefore possible that the medical histories of the controls were not appropriate for our study. Selection bias may also have occurred as all the cases were obtained from the database of a reference hospital. We therefore may have missed out on patients with less severe BP, who remained under the care of their primary care physicians or of a smaller hospital without an electronic medical record system. This may have led to an overestimation of the associations observed.
In conclusion, we found an association between certain neurological conditions and BP in our population, supporting previous reports in the literature. Nevertheless, it has not yet been established whether this association is purely statistical or if there is a causal link. One plausible cause would be immunological cross-reactivity, whereby the neurological event would cause destruction of the blood-brain barrier and consequent exposure of the neuronal isoform BP230, triggering the immune system. Whatever the case, more studies, probably of a cohort nature, are needed to estimate the relative risk of the development of BP associated with each of these comorbidities.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Casas-de-la-Asunción E, Ruano-Ruiz J, Rodríguez-Martín A, Vélez García-Nieto A, Moreno-Giménez J. Asociación de penfigoide y enfermedad neurológica: estudio de casos y controles. Actas Dermosifiliogr. 2014;105:860–865.