It is important to identify subgroups within the general population that have an elevated risk of developing cutaneous melanoma because preventive and early-detection measures are useful in this setting. The findings of most studies that have evaluated risk factors for cutaneous melanoma are of limited application in Spain because the populations studied have different pigmentary traits and are subject to different environmental factors.
ObjectiveTo identify the phenotypic characteristics and amount of exposure to sunlight that constitute risk factors for cutaneous melanoma in the population of the Autonomous Community of Valencia, Spain.
MethodsWe performed a multicenter observational case-control study. In total, the study included 242 patients with melanoma undergoing treatment in 5 hospitals and 173 controls enrolled from among the companions of the patients between January 2007 and June 2008. The information was collected by means of a standardized, validated questionnaire. The odds ratio (OR) was calculated for each variable and adjusted using a multiple logistic regression model.
ResultsThe risk factors found to be statistically significant were skin phototypes I and II, blond or red hair, light eye color, abundant melanocytic nevi, and a personal history of actinic keratosis or nonmelanoma skin cancer. After the multivariate analysis, only blond or red hair (OR=1.9), multiple melanocytic nevi (OR=3.1), skin phototypes i and ii (OR=2.1), and a personal history of actinic keratosis (OR=3.5) or nonmelanoma skin cancer (OR=8.1) maintained significance in the model as independent predictive variables for melanoma.
ConclusionsOur study supports the importance of certain factors that indicate genetic predisposition (hair color and skin phototype) and environmental factors associated with exposure to sunlight. Patients with multiple acquired melanocytic nevi and patients with markers of chronic skin sun damage (actinic keratosis and nonmelanoma cancer) presented a significant increase in risk.
En la población general es importante identificar aquellos subgrupos con un riesgo elevado de padecer un melanoma cutáneo, por la posibilidad de aplicar medidas preventivas y de detección temprana de la enfermedad. La mayoría de los estudios realizados que evalúan estos factores de riesgo tienen una aplicabilidad limitada en nuestro medio, debido a que las poblaciones estudiadas están sometidas a distintos factores ambientales y los rasgos pigmentarios son diferentes.
ObjetivosIdentificar qué características fenotípicas individuales y relacionadas con la exposición solar son factores de riesgo para desarrollar un melanoma cutáneo en la población de la Comunidad Valenciana.
MétodosRealizamos un estudio multiinstitucional observacional de casos y controles. Fueron incluidos 242 casos de melanoma incidentes tratados en 5 hospitales, y 173 controles recogidos entre los acompañantes de los pacientes entre enero de 2007 y junio de 2008. La información fue recogida mediante un cuestionario estandarizado y validado. Fue calculada la odds ratio (OR) para cada variable y ajustada mediante regresión logística múltiple.
ResultadosLos fototipos i-ii, el color de pelo rubio o pelirrojo, el color de ojos claro, la presencia de abundantes nevos melanocíticos y los antecedentes personales de queratosis actínicas o de cáncer cutáneo no melanoma fueron los factores de riesgo estadísticamente significativos. Tras el estudio multivariado solo el color de pelo rubio o pelirrojo (OR=1,9), la presencia de múltiples nevos melanocíticos (OR=3,1), los fototipos i-ii (OR=2,1) y los antecedentes personales de queratosis actínicas (OR=3,5) o de cáncer cutáneo no melanoma (OR=8,1) se mantuvieron en el modelo como las variables predictivas independientes relacionadas con el desarrollo de melanoma.
ConclusionesNuestro estudio apoya la importancia de una serie de factores que indican predisposición genética (color de pelo y fototipo) y ambientales relacionados con la exposición solar. Los pacientes con múltiples nevos melanocíticos adquiridos, y también aquellos con marcadores de daño solar crónico (queratosis actínicas y cáncer cutáneo no melanoma), presentaron un significativo aumento del riesgo.
Cutaneous melanoma is a malignant tumor that originates from skin melanocytes. Its incidence and mortality have increased progressively in recent decades.1 The risk of metastasis depends largely on tumor thickness, which, with more or less variability, is related to how early the lesion is diagnosed.2 Early detection and treatment are very important in melanoma as cure rates of over 90% can be achieved if the tumor is detected in its early stages.2,3
It is important to identify subgroups within the general population that have an elevated risk of melanoma in order to apply preventive measures aimed at early detection and identify risk characteristics or factors that can be modified where possible.
Most of the studies that have analyzed risk factors in cutaneous melanoma have been conducted in English-speaking countries and in the north of Europe.1,4,5 Their findings, however, are of limited application in Spain because the populations studied are exposed to different environmental factors (e.g., UV radiation) and have different pigmentary traits. Some studies, however, have been performed in southern European countries with a lower incidence of melanoma.6,7 Several meta-analyses of the most important studies conducted to date on risk factors for cutaneous melanoma have contributed to estimating the risk associated with each of the factors identified.8–10 To date, only 2 studies—conducted in 116 patients with melanoma in Granada in southern Spain–have analyzed risk factors associated with melanoma in the Spanish population.11,12 The main risk factors identified were skin phenotype (hair color, number of melanocytes, phototype) and sun exposure levels.
The aim of the present study was to perform a case-control study to identify the risk factors for cutaneous melanoma in the population of the Autonomous Community of Valencia in Spain and to investigate their association with individual phenotypes and sun exposure.
Participants and MethodsWe performed a multicenter prospective observational case-control study. For the study group, we included all incident cases of melanoma treated in the dermatology departments of the following hospitals in the Autonomous Community of Valencia: Instituto Valenciano de Oncología (IVO), Consorcio Hospital General Universitario de Valencia (CHGUV), Hospital de la Plana de Villarreal (HLPC), Hospital General Universitario de Alicante (HGUA), and Hospital Clínico San Juan de Alicante (HCSJA). All patients with histologically confirmed melanoma who received definitive treatment at any of the participating departments between January 2007 and June 2008 were included. We limited the study to the 3 most common forms of melanoma: superficial spreading melanoma, lentigo maligna melanoma, and nodular melanoma. Acral lentiginous melanoma was not included as it has different risk factors. The study was approved by the ethics committee of the IVO.
The controls were recruited from individuals accompanying patients on their visits to the dermatology departments, with the exclusion of relatives of patients with melanoma; the controls were from the same geographic area as the cases.
Informed consent was obtained from all the cases and controls. Data were collected from all participants using a standardized questionnaire administered in a face-to-face interview. The investigators were not blinded to whether the individuals being interviewed were cases or controls. The questionnaire was adapted from the validated questionnaire used in the Queensland Familial Melanoma Project.13
Information was collected on the following:
- 1
Epidemiological characteristics: sex, age, place of birth, level of education.
- 2
Phenotypic characteristics: skin phototype (I/II vs III/IV), eye color (dark vs light), hair color (brown/black vs blond/red), number of common melanocytic nevi (none/some vs moderate/many), number of freckles on the face (none/some vs moderate/many), and number of freckles on the arms (none/some vs moderate/many). Melanocytic nevus and freckle counts were recorded on the diagrams used in the Queensland Familial Melanoma Project.13
- 3
Environmental factors: sun exposure, including questions on lifetime exposure. The questionnaire included a table to record hours of maximum sun exposure during childhood, adolescence, and adulthood. Data were collected on occupational sun exposure (mean hours of exposure during the week and at weekends); nonoccupational sun exposure (mean hours of exposure during the week, at weekends, and during holidays); history of severe sunburn (0-1, 2-4, or ≥5 episodes); use of sunbeds (0, 1-10, 11-20, 21-60, >60 uses); age of first exposure to UV rays from a sunbed (<20, 20-40, ≥41 years); and use of sunscreen (yes or no).
- 4
Personal history of actinic keratosis or nonmelanoma skin cancer (yes or no) and history of melanoma or other cancers in first-degree relatives (yes or no).
We included 242 patients diagnosed with cutaneous melanoma at the participating centers, specifically, 137 from the IVO (56.6%), 56 from the CHGUV (23.1%), 26 from the HGUA (10.7%), 13 from the HCSJA (5.4%), and 10 from the HLPC (4.1%)
The group consisted of 128 men (52.9%) and 114 women (47.1%). The median age was 57 years (interquartile range [IQR], 43-69 years). In total, 277 (93.8%) were Spanish and 15 (6.2%) were foreign.
ControlsWe included 173 controls. Of these, 120 (70.7%) were spouses of patients with cutaneous melanoma, 24 (14.2%) were friends, and 25 (15.1%) were individuals who had accompanied patients consulting for a condition other than cutaneous melanoma.
There were 61 men (35.3%) and 112 women (64.7%). The median age was 54 years (IQR, 42-64 years) and 98.2% were Spanish.
The χ2 test of homogeneity was used to analyze similarities in the main study variables between cases and controls, and the Fisher exact test was used for variables with an expected frequency of 5 or less. Odds ratios (ORs) were calculated by univariate and multivariate logistic regression analyses. The multivariate analysis included significant risk factors identified in the univariate study, with adjustment for sex. The weighting of each risk factor was measured using the Wald χ2 statistic for each of the factors in the logistic regression model. Factors with the highest χ2 values were considered to be the most significant factors in each model. The Bonferroni correction for multiple comparisons was applied to P values used to identify between-group heterogeneity. Statistical significance was set at a value of P<.05. Statistical analyses were performed using the SPPS statistical package (version 19).
ResultsOf the 415 cases and controls studied, 189 (45.5%) were men and 226 (54.5%) were women, with a median age of 56 years (IQR, 43-67 years). A statistically significant difference (P=.002) was found for the distribution of sexes between cases and controls. There were more women in the control group (64.7%) and more men in the melanoma group (52.9%). No significant differences were found for age, classified as ≤40 or >40 years. The proportion of individuals aged 41 years or older was 79.5% in the melanoma group and 79.8% in the control group.
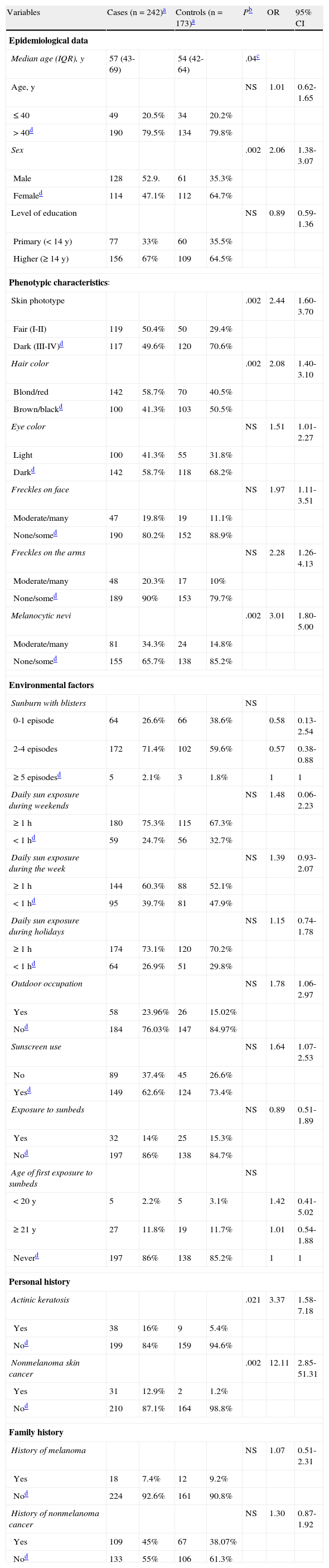
Table 1 shows the distribution of the study variables. In the phenotype category, significant differences were found for skin phototypes I and II, blond or red hair, light-colored eyes, and the presence of multiple melanocytic nevi. While 50.4% of the patients with melanoma had skin phototypes I or II, only 29.4% of the controls did. Those with phototypes I or II had a 2-fold higher odds (OR, 2.44; 95% CI, 1.60-3.70) of developing melanoma compared to those with phototypes III or IV. Blond or red hair was associated with an OR for developing melanoma of 2.08 (95% CI, 1.40-3.10) as compared with brown or black hair. The ratio for light-colored eyes compared to dark-colored eyes was 1.51 (95% CI, 1.01-2.27). Individuals with multiple melanocytic nevi had an 3-fold higher odds (OR, 3.01; 95% CI, 1.80-5.00) of developing melanoma compared to those with few or no nevi. No significant between-group differences were found for abundant freckling on the face or arms.
Differences in Epidemiological Data, Phenotypic Characteristics, Environmental Factors, and Personal and Family History Between Patients With Melanoma and Controls (Univariate Analysis).
| Variables | Cases (n=242)a | Controls (n=173)a | Pb | OR | 95% CI | ||
| Epidemiological data | |||||||
| Median age (IQR), y | 57 (43-69) | 54 (42-64) | .04c | ||||
| Age, y | NS | 1.01 | 0.62-1.65 | ||||
| ≤40 | 49 | 20.5% | 34 | 20.2% | |||
| >40d | 190 | 79.5% | 134 | 79.8% | |||
| Sex | .002 | 2.06 | 1.38-3.07 | ||||
| Male | 128 | 52.9. | 61 | 35.3% | |||
| Femaled | 114 | 47.1% | 112 | 64.7% | |||
| Level of education | NS | 0.89 | 0.59-1.36 | ||||
| Primary (<14 y) | 77 | 33% | 60 | 35.5% | |||
| Higher (≥14 y) | 156 | 67% | 109 | 64.5% | |||
| Phenotypic characteristics: | |||||||
| Skin phototype | .002 | 2.44 | 1.60-3.70 | ||||
| Fair (I-II) | 119 | 50.4% | 50 | 29.4% | |||
| Dark (III-IV)d | 117 | 49.6% | 120 | 70.6% | |||
| Hair color | .002 | 2.08 | 1.40-3.10 | ||||
| Blond/red | 142 | 58.7% | 70 | 40.5% | |||
| Brown/blackd | 100 | 41.3% | 103 | 50.5% | |||
| Eye color | NS | 1.51 | 1.01-2.27 | ||||
| Light | 100 | 41.3% | 55 | 31.8% | |||
| Darkd | 142 | 58.7% | 118 | 68.2% | |||
| Freckles on face | NS | 1.97 | 1.11-3.51 | ||||
| Moderate/many | 47 | 19.8% | 19 | 11.1% | |||
| None/somed | 190 | 80.2% | 152 | 88.9% | |||
| Freckles on the arms | NS | 2.28 | 1.26-4.13 | ||||
| Moderate/many | 48 | 20.3% | 17 | 10% | |||
| None/somed | 189 | 90% | 153 | 79.7% | |||
| Melanocytic nevi | .002 | 3.01 | 1.80-5.00 | ||||
| Moderate/many | 81 | 34.3% | 24 | 14.8% | |||
| None/somed | 155 | 65.7% | 138 | 85.2% | |||
| Environmental factors | |||||||
| Sunburn with blisters | NS | ||||||
| 0-1 episode | 64 | 26.6% | 66 | 38.6% | 0.58 | 0.13-2.54 | |
| 2-4 episodes | 172 | 71.4% | 102 | 59.6% | 0.57 | 0.38-0.88 | |
| ≥5 episodesd | 5 | 2.1% | 3 | 1.8% | 1 | 1 | |
| Daily sun exposure during weekends | NS | 1.48 | 0.06-2.23 | ||||
| ≥1h | 180 | 75.3% | 115 | 67.3% | |||
| <1hd | 59 | 24.7% | 56 | 32.7% | |||
| Daily sun exposure during the week | NS | 1.39 | 0.93-2.07 | ||||
| ≥1h | 144 | 60.3% | 88 | 52.1% | |||
| <1hd | 95 | 39.7% | 81 | 47.9% | |||
| Daily sun exposure during holidays | NS | 1.15 | 0.74-1.78 | ||||
| ≥1h | 174 | 73.1% | 120 | 70.2% | |||
| <1hd | 64 | 26.9% | 51 | 29.8% | |||
| Outdoor occupation | NS | 1.78 | 1.06-2.97 | ||||
| Yes | 58 | 23.96% | 26 | 15.02% | |||
| Nod | 184 | 76.03% | 147 | 84.97% | |||
| Sunscreen use | NS | 1.64 | 1.07-2.53 | ||||
| No | 89 | 37.4% | 45 | 26.6% | |||
| Yesd | 149 | 62.6% | 124 | 73.4% | |||
| Exposure to sunbeds | NS | 0.89 | 0.51-1.89 | ||||
| Yes | 32 | 14% | 25 | 15.3% | |||
| Nod | 197 | 86% | 138 | 84.7% | |||
| Age of first exposure to sunbeds | NS | ||||||
| <20y | 5 | 2.2% | 5 | 3.1% | 1.42 | 0.41-5.02 | |
| ≥21y | 27 | 11.8% | 19 | 11.7% | 1.01 | 0.54-1.88 | |
| Neverd | 197 | 86% | 138 | 85.2% | 1 | 1 | |
| Personal history | |||||||
| Actinic keratosis | .021 | 3.37 | 1.58-7.18 | ||||
| Yes | 38 | 16% | 9 | 5.4% | |||
| Nod | 199 | 84% | 159 | 94.6% | |||
| Nonmelanoma skin cancer | .002 | 12.11 | 2.85-51.31 | ||||
| Yes | 31 | 12.9% | 2 | 1.2% | |||
| Nod | 210 | 87.1% | 164 | 98.8% | |||
| Family history | |||||||
| History of melanoma | NS | 1.07 | 0.51-2.31 | ||||
| Yes | 18 | 7.4% | 12 | 9.2% | |||
| Nod | 224 | 92.6% | 161 | 90.8% | |||
| History of nonmelanoma cancer | NS | 1.30 | 0.87-1.92 | ||||
| Yes | 109 | 45% | 67 | 38.07% | |||
| Nod | 133 | 55% | 106 | 61.3% | |||
Abbreviations: IQR, interquartile range; NS, not significant; OR, odds ratio.
On analyzing personal and family history, significant differences were found between cases and controls for a personal history of actinic keratosis and of nonmelanoma skin cancer. Sixteen percent of patients with melanoma and just 5.4% of controls had a personal history of actinic keratosis (OR, 3.37; 95% CI, 1.58-7.18). The OR for developing melanoma in patients with a history of nonmelanoma skin cancer was 12.10 (95% CI, 2.85-58.45), as compared to those without such a history.
No significant differences were found between cases and controls for any of the environmental factors analyzed (sunscreen use; occupational sun exposure; nonoccupational sun exposure during the week, at weekends, or during holidays; sunbed use; age of first exposure to UV rays from a sunbed; or history of severe sunburn).
There were also no significant differences detected for a family history of melanoma or other type of cancer.
Based on the Wald test results, the following factors, shown by order of significance, were most closely related to the development of melanoma: multiple melanocytic nevi, skin phototypes I and II, a personal history of nonmelanoma skin cancer, blond or red hair, and a personal history of actinic keratosis.
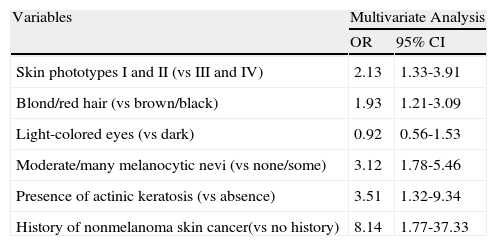
In the multivariate analysis adjusted for sex, the following factors retained their value as independent predictors of melanoma: hair color, number of melanocytic nevi, skin phototype, and a personal history of actinic keratosis or nonmelanoma skin cancer (Table 2). Patients with a personal history of nonmelanoma skin cancer had the highest odds of developing cutaneous melanoma (OR, 8.14; 95% CI, 1.77-37.33).
Risk Factors for Cutaneous Melanoma: Multivariate Study Adjusted for Age.
| Variables | Multivariate Analysis | |
| OR | 95% CI | |
| Skin phototypes I and II (vs III and IV) | 2.13 | 1.33-3.91 |
| Blond/red hair (vs brown/black) | 1.93 | 1.21-3.09 |
| Light-colored eyes (vs dark) | 0.92 | 0.56-1.53 |
| Moderate/many melanocytic nevi (vs none/some) | 3.12 | 1.78-5.46 |
| Presence of actinic keratosis (vs absence) | 3.51 | 1.32-9.34 |
| History of nonmelanoma skin cancer(vs no history) | 8.14 | 1.77-37.33 |
The present study has a number of limitations that need to be taken into account when interpreting its results. First, it is possible that bias was introduced during the recruitment of controls, who were slightly less numerous than cases. Matching was not used in the selection process, but we did adjust for sex in the multivariate analysis as this was the epidemiological variable for which the greatest difference was detected between cases and controls. We also tried to collect information on as many potential confounders as possible to adjust for these during analysis. Most of the controls (70%) were the spouses of patients with melanoma. This might have influenced the significance of certain results, particularly with respect to sun exposure, as spouses may have had similar sun exposure habits. It is important to note that all the data were collected using a standardized questionnaire (although this was administered by different observers) and that classification bias may have been introduced during the physical examination. Recall bias is assumed to have occurred in the reporting of hours of sun exposure and number of serious sunburn episodes. Nonetheless, the robustness of our results is strengthened by the fact that Bonferroni adjustment was used for multiple corrections. This method minimizes the likelihood of significant findings being due to chance when analyzing large numbers of variables.
Epidemiological studies of populations in northern Europe have established that individuals with light-colored eyes and fair skin and hair have an increased risk of cutaneous melanoma compared to those with darker features.14–16 Our results show that fair skin (phototypes I and II) and fair hair but not light-colored eyes were the only statistically significant risk factors for developing melanoma in a Mediterranean population. These findings provide further evidence of the importance of individual pigmentary traits as risk factors for cutaneous melanoma, even in the Mediterranean population, who have darker skin and a lower incidence of melanoma than their counterparts in northern Europe or in English-speaking countries.Several authors have reported blond and red hair to be a risk factor for cutaneous melanoma,17,18 but for others, this trait loses its significance following correction for confounding factors.19–21
In our series, light-colored eyes was a risk factor for melanoma in the univariate study, but failed to maintain its significance in the multivariate analysis. We also found no significant differences between cases and controls in terms of number of freckles, but it has been postulated that all these traits are interrelated and are probably dependent on photosensitivity in individuals with skin phototypes I and II.11
The risk of developing cutaneous melanoma in children and adults was reported to depend strongly on the number of melanocytic nevi.8 Coinciding with the findings of other studies, our study showed an elevated risk of cutaneous melanoma in patients with more melanocytic nevi.
There are conflicting data on the association between chronic or intermittent sun exposure and the risk of cutaneous melanoma. Several studies have suggested that intermittent sun exposure is associated with a higher risk.9,18,19,22 The results of the present study suggest that an increased risk of cutaneous melanoma might be related to intense sun exposure in patients with a history of sunburn, but the association was not significant. Our study, like others conducted in the Mediterranean area6,20,23 did not detect an association between sunburn and melanoma risk. The fact that these results contradict those from studies performed in other areas supports the hypothesis that the influence of sunburn on the risk of cutaneous melanoma might be linked to latitude, darker skin, or even different sun exposure practices (e.g., greater use of protection). Nonetheless, we cannot rule out the possibility that patients might have underestimated the number of occasions on which they had been sunburnt.
We also found an increased though not statistically significant risk of cutaneous melanoma in patients with chronic occupational sun exposure. This might be due, at least in part, to the fact that we analyzed a combination of types of melanoma; lentigo maligna, for example, is associated with different epidemiological characteristics to superficial spreading melanoma and nodular melanoma.
We did not find significant differences between cases and controls for daily sun exposure during the week, at weekends, or during holidays, possibly because our sample was too small to evaluate potential differences between these groups. We also believe that sun exposure levels may be difficult to measure objectively in our setting as the population is constantly exposed to intense sunlight and it is therefore difficult to distinguish between chronic exposure and intermittent recreational exposure.
There is a clear link between chronic sun exposure and the risk of developing cutaneous melanoma if we consider that melanoma is associated with a history of actinic keratosis and nonmelanoma skin cancer, 2 biologic responses that are closely related to high cumulative sun exposure.24 In our series, we also found an increased risk of cutaneous melanoma in patients with a history of actinic keratosis and nonmelanoma skin cancer. These factors have been found to be important risk markers in several studies, confirming the important role of cumulative sun exposure in the etiology and pathogenesis of cutaneous melanoma.25
We did not observe a significant association between increased melanoma risk and failure to use sunscreen, and similar results have been described in other studies. Holman et al.26 and Osterlind et al.,27 for example, found no modification of risk, while Beitner et al.14 reported a paradoxical association between increased risk of cutaneous melanoma and frequent sunscreen, possibly explained by longer exposure to sun among sunscreen users or to the fact that these products contain a chemical agent that might increase the risk of melanoma. Nonetheless, it is important to note that many of the studies that have analyzed this risk factor included individuals who did not use effective sunscreens during childhood and did not control for confounders such as type of sun exposure or type of sunscreen. One meta-analysis reported an association between an increased risk of cutaneous melanoma and sunscreen use in countries at latitudes at over 40° from the equator.28 The association was not observed for countries closer to the equator, but the differences might be due more to skin type than to differences in UV radiation.
We found no association between melanoma risk and sunbed use, but only a small percentage of the individuals in our series (13.7%) had ever used a sunbed. The earliest results reported for the association between exposure to UV rays from sunbeds and the risk of cutaneous melanoma were the subject of debate. One multicenter European study, performed in 5 countries, for example, found no significant association.29 However, a more recent meta-analysis reported that sunbed use was associated with a risk of 1.15 (95% CI, 1.00-1.31).30 A dose-response association was not observed, but a significant risk was seen for individuals who had first used a sunbed before the age of 35 years. The findings led the World Health Organization to declare sunbeds carcinogenic. Further studies should perhaps be conducted in Spain to shed light on the conflicting results detected in our series.
In conclusion, the findings of this case-control study of the risk factors for cutaneous melanoma support the importance of certain factors that indicate genetic predisposition (hair color and skin phototype) and environmental factors associated with exposure to sunlight. Patients with multiple acquired melanocytic nevi and patients with markers of chronic skin sun damage (actinic keratosis and nonmelanoma cancer) had a significant increase in risk. The fact that a personal history of nonmelanoma skin cancer was found to be a significant risk factor highlights the importance of physical examination and education in such patients to aid the early detection of cutaneous melanoma.
FundingThis study was supported by a research project development grant (AP-098/07) from the Ministry of Health of the Autonomous Government of Valencia, Spain.
Conflict of InterestsThe authors declare that they have no conflicts of interest.
Please cite this article as: Ballester I, et al. Estudio multicéntrico de casos y controles sobre factores de riesgo de desarrollar un melanoma cutáneo en la Comunidad Valenciana. Actas Dermosifiliogr. 2012;103:790-7.