Enoxaparin is a low-molecular-weight heparin used in the prevention and treatment of pulmonary thromboembolism and other thrombotic disorders. The most common adverse reactions to enoxaparin are ecchymosis, skin necrosis, urticaria, angioedema, and eczema. The first 2 cases of bullous hemorrhagic dermatosis in areas distant from heparin injection sites were described in 2006.
We present the cases of 2 men, aged 68 and 78 years, with progressive, advanced-stage lung cancer, who consulted with bullous hemorrhagic lesions without associated symptoms. Both patients reported that the lesions had appeared after initiation of heparin therapy at therapeutic doses.
In our review of the literature, we found just 7 cases of heparin-induced bullous hemorrhagic dermatosis. We report a further 2 cases, caused by enoxaparin, in which treatment was continued and in which the lesions resolved in 2 to 3 weeks.
La enoxaparina pertenece al grupo de heparinas de bajo peso molecular. Se utiliza en el manejo terapéutico y profiláctico del tromboembolismo venoso pulmonar y otros cuadros trombóticos. Las reacciones adversas cutáneas más frecuentes son equimosis, necrosis cutánea, urticaria, angioedema y eccema. En el año 2006 se describieron los primeros casos de dermatosis ampollosa hemorrágica a distancia por heparina.
Presentamos dos varones de 68 y 78 años, con carcinomas de pulmón en estadios avanzados y en progresión, que consultaban por lesiones ampollosas hemorrágicas, sin otra sintomatología acompañante. Ambos referían la aparición de las lesiones tras comenzar con la administración de heparina a dosis terapéuticas.
En la literatura revisada solo hemos encontrado descritos 7 casos de dermatosis ampollosa hemorrágica por heparina. Aportamos dos nuevos casos por enoxaparina, en los que se mantuvo el tratamiento y se resolvió el cuadro en dos a tres semanas.
Enoxaparin is a low-molecular-weight heparin (LMWH) that is used for the prevention and treatment of pulmonary venous thromboembolism and other thrombotic disorders, such as myocardial infarction. The drug is administered once or twice daily, depending on the indication. The most common complication associated with these anticoagulants is bleeding. Adverse skin reactions may be localized or generalized and can be immediate or delayed; the most common are ecchymoses, skin necrosis, urticaria, angioedema, and eczema.2,3
Seven cases of hemorrhagic bullous dermatosis occurring in areas distant from the site of injection have been reported in patients receiving treatment with various LMWHs (dalteparin,4 enoxaparin,5,6 tinzaparin4,6) or unfractionated heparins (calcium heparin4). We present 2 new cases related to the administration of enoxaparin.
Patient 1Our first patient was a 68-year-old man with a past history of systemic hypertension, diabetes mellitus, hypercholesterolemia and a progressive, stage IV undifferentiated carcinoma, probably of pulmonary origin, diagnosed in 2009 and for which he had received 3 cycles of palliative chemotherapy with cisplatin and etoposide. He was also on treatment with prednisone, metformin, enalapril, and simvastatin. During his most recent admission he had been started on treatment with enoxaparin, 60mg subcutaneously every 12hours, for superior vena cava syndrome. Fifteen days later he came to the emergency department for asymptomatic, hemorrhagic vesicular-bullous lesions on the dorsum of the hand, around the lower jaw and in the malar region on the right side. The lesions had appeared 1 week earlier and had been treated with silver nitrate. The patient did not report bleeding in any other area of the body.
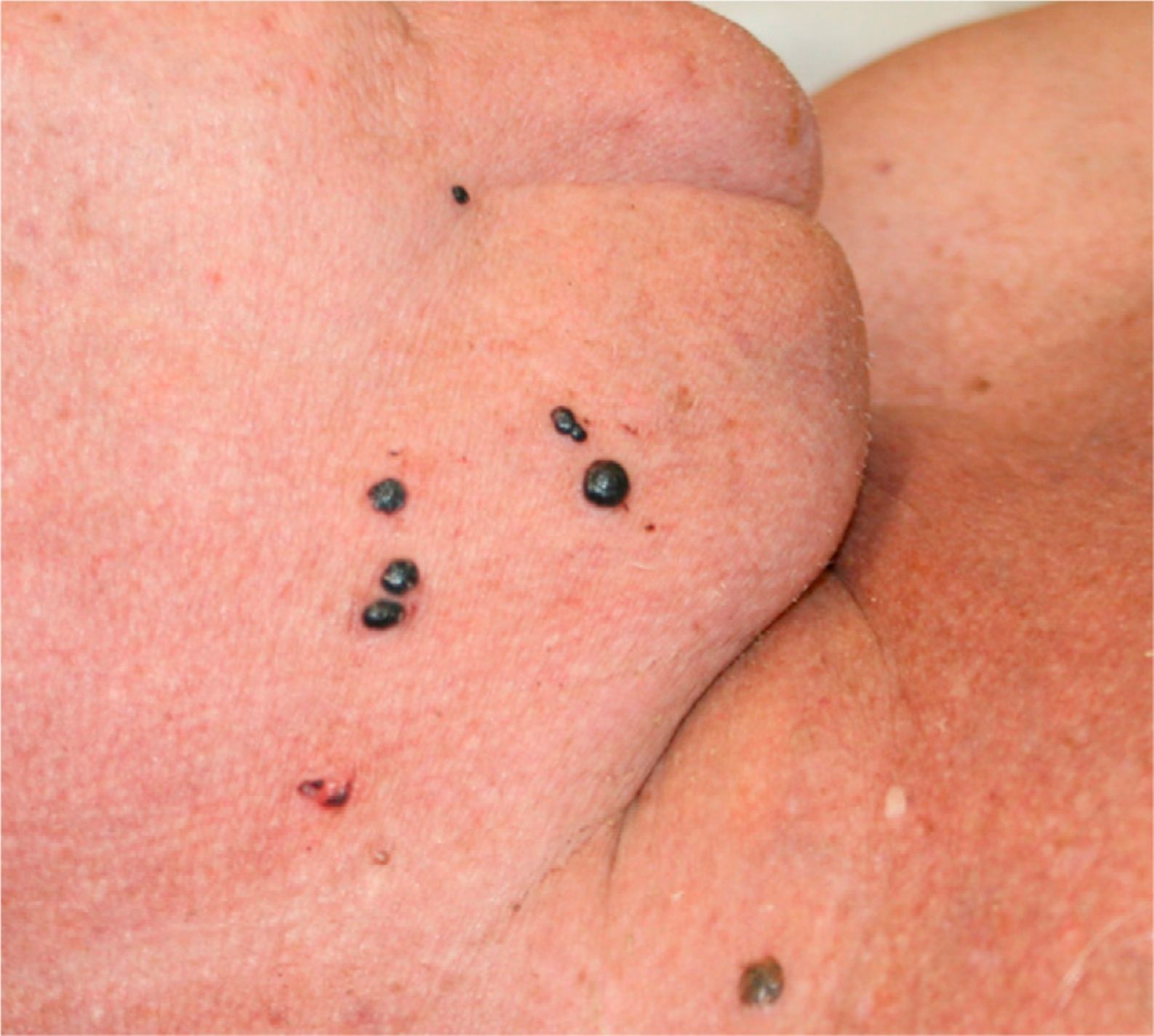
On physical examination there was a tense, noninflammatory blood-filled vesicle of 7mm in diameter on the dorsum of the hand right and about 15 vesicular lesions of the same characteristics, measuring between 2 and 8mm in diameter, in groups on the right side of the neck and right malar region (Fig. 1).
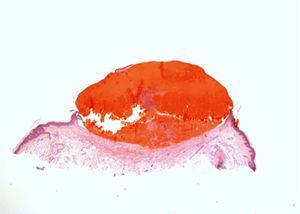
Laboratory tests were within the normal ranges for platelet count and coagulation studies (prothrombin time [PT], prothrombin activity, international normalized ratio [INR], thromboplastin time, and fibrinogen level). A biopsy taken from the lesion on the dorsum of the hand revealed a purulent, fibrin-covered necrotic ulcer, with fibrosis and granulation tissue in its base and residual reparative epithelial changes.
Patient 2The second patient was a 78-year-old man with a past history of recently diagnosed stage IV non-small-cell lung cancer with lung and liver metastases, ischemic heart disease, and a biological mitral valve replacement in 2006. He was on treatment with prednisone, codeine, ticlopidine, omeprazole, atorvastatin, nitroglycerine, and enoxaparin, 80mg by subcutaneous injection every 12hours. We were asked to evaluate asymptomatic hemorrhagic skin lesions that had developed 10 days earlier on both knees, the right forearm, and the facial region. The patient reported that 10 days before the lesions appeared, he had started treatment with enoxaparin. There were no other symptoms.
Examination revealed dry, tense hemorrhagic vesicles of up to 5mm in diameter occurring in groups on the right knee and forearm and an isolated vesicle in the facial region.
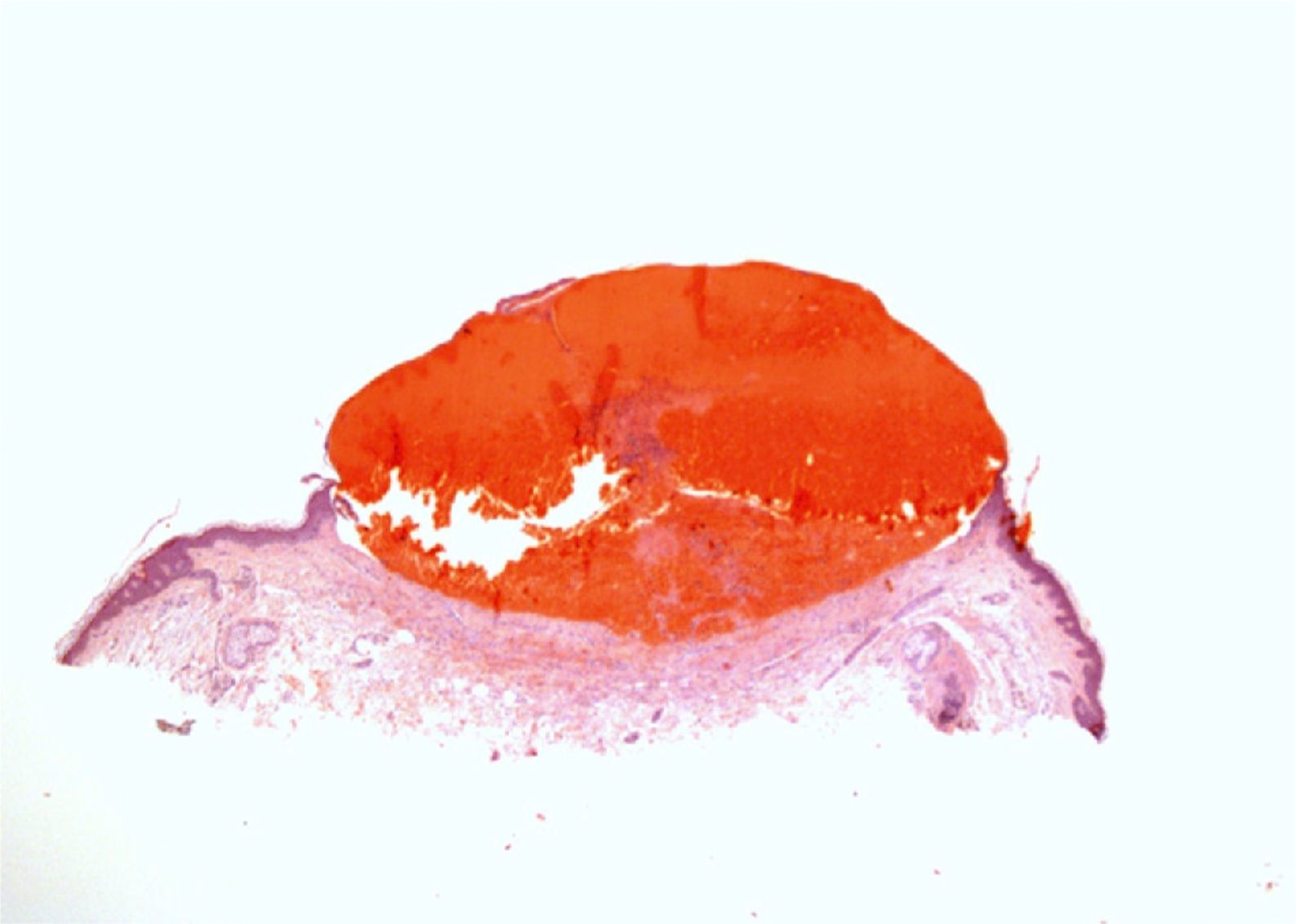
A complete blood count, biochemistry, and coagulation studies were performed, as well as biopsy of a lesion on the right forearm. Histology revealed an intraepidermal vesicle containing blood, fibrin and occasional neutrophils. There was red blood cell extravasation in the underlying dermis and a mixed inflammatory infiltrate with neutrophils; there were no signs of vasculitis (Fig. 2). The results of the complete blood count, including platelet count, and coagulation studies (PT, INR, prothrombin activity, thromboplastin time, fibrinogen, antithrombin III, protein C, and protein S) were within normal limits.
Treatment was continued with enoxaparin at the recommended dose in both cases, and no topical or systemic treatments were added. The lesions resolved without sequelae in 2 to 3 weeks.
DiscussionEnoxaparin, a LMWH obtained by depolymerization of standard heparin, is used both for prophylaxis and for the treatment of thrombotic phenomena. It acts by binding to antithrombin III, causing inhibition of factor Xa. Bleeding is the most common complication of enoxaparin therapy.
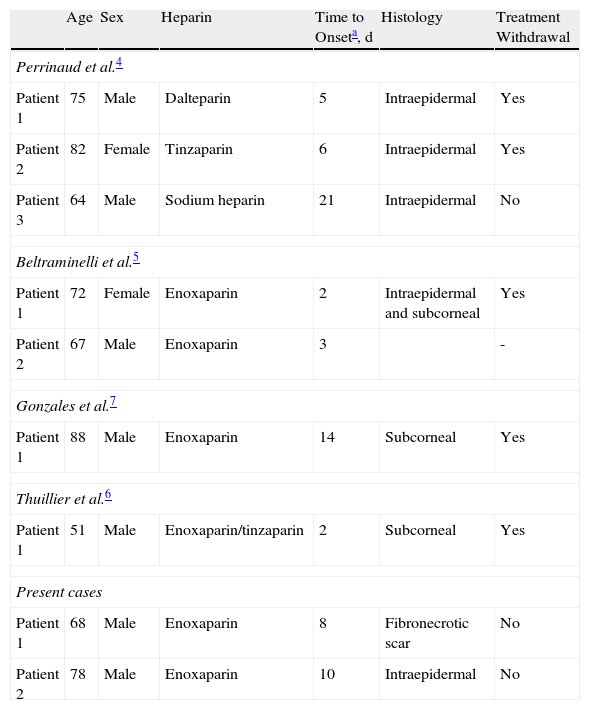
In 2006, Perrinaud et al.4 were the first to describe patients with hemorrhagic bullous lesions occurring at a distance from the site of injection and that were related to the administration of 2 types of heparin molecule: LMWHs (dalteparin, tinzaparin) and an unfractionated heparin (calcium heparin). Those authors described 3 patients with eruptions of tense, noninflammatory hemorrhagic blisters distributed in groups at a distance from the site of subcutaneous injection of the heparin.1 Later, in 2009, Beltraminelli et al.,5 Thuillier et al.,6 and Gonzales et al.7 reported a total of 4 cases similar to those described by Perrinaud et al. and that were associated with the administration of enoxaparin sodium and tinzaparin (Table 1).
Heparin-induced Hemorrhagic Bullous Dermatosis at a Site Distant From the Injection.
| Age | Sex | Heparin | Time to Onseta, d | Histology | Treatment Withdrawal | |
| Perrinaud et al.4 | ||||||
| Patient 1 | 75 | Male | Dalteparin | 5 | Intraepidermal | Yes |
| Patient 2 | 82 | Female | Tinzaparin | 6 | Intraepidermal | Yes |
| Patient 3 | 64 | Male | Sodium heparin | 21 | Intraepidermal | No |
| Beltraminelli et al.5 | ||||||
| Patient 1 | 72 | Female | Enoxaparin | 2 | Intraepidermal and subcorneal | Yes |
| Patient 2 | 67 | Male | Enoxaparin | 3 | - | |
| Gonzales et al.7 | ||||||
| Patient 1 | 88 | Male | Enoxaparin | 14 | Subcorneal | Yes |
| Thuillier et al.6 | ||||||
| Patient 1 | 51 | Male | Enoxaparin/tinzaparin | 2 | Subcorneal | Yes |
| Present cases | ||||||
| Patient 1 | 68 | Male | Enoxaparin | 8 | Fibronecrotic scar | No |
| Patient 2 | 78 | Male | Enoxaparin | 10 | Intraepidermal | No |
The etiology and pathogenesis of these lesions is unknown. In our cases there were no alterations of routine coagulation studies. Two of the patients reported by Perrinaud et al.4 were also taking acetylsalicylic acid and the third was on dipyridamole (an antiplatelet drug) concomitantly with the heparin. Our second patient was also taking ticlopidine.
As in the 2 cases we present, the histopathology of lesions in the previously published cases was nonspecific: blood-filled intraepidermal or subcorneal vesicles and blisters with no signs of vasculitis or capillary thrombosis. Subepidermal spongiosis and the presence of eosinophils were only observed in the patient described by Thuillier et al.6
Management of the lesions and the decision on whether to interrupt heparin treatment varied in the previously published cases. Perrinaud et al.4 stated that they replaced the heparin with oral anticoagulants in their first patient, but the patient died 7 days later due to hemorrhagic stroke, and thus the clinical course could not be studied. In their second patient, they discontinued tinzaparin and the lesions resolved at 10 days; in their third patient the lesions resolved even with no change in therapy. Beltraminelli et al.5 only described the clinical course of 1 of their patients, in whom they substituted the heparin with an oral anticoagulant; the lesions resolved within 2 weeks. In the patients reported by Gonzales et al.7 and Thuiller et al.6 the authors decided to withdraw the heparin and the lesions disappeared within a few weeks. We decided to maintain heparin therapy in our patients and the lesions resolved without treatment within 2 to 3 weeks. As occurred in our patients, the lesions in at least 3 of the patients described in the literature resolved despite continuing heparin treatment.
Enoxaparin belongs to the group of LMWHs, which also includes bemiparin, dalteparin, nadroparin, and tinzaparin. In the literature reviewed, enoxaparin was the heparin most frequently associated with the appearance of hemorrhagic blisters—it was being administered in 6 of the 9 cases reported, including ours. The more frequent use of enoxaparin may be due to the influence on prescribers of its ease of administration, the absence of a need for blood tests to monitor therapy, and its safety profile.
In conclusion, heparin-induced hemorrhagic bullous dermatosis occurring at a distance from the site of injection is a rare adverse skin reaction, although the fact that it is typically self-limiting, even without discontinuation of the medication, could mean that it is an underdiagnosed phenomenon or one that is under-reported in the literature. Only 7 cases have been reported prior to the cases we describe. We would like to draw attention to the favourable clinical course of these eruptions in our patients and in those described in the literature, even without treatment modification, and to the absence of associated complications.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Villanueva CA, et al. Dermatosis ampollosa hemorrágica a distancia; dos nuevos casos por enoxaparina y revisión de la literatura. Actas Dermosifiliogr. 2012;103:816-9.