Basal cell carcinoma (BCC) is common cutaneous malignancy.
AimsTo examine the expression patterns of CD10, p63, BCL-2, and epithelial membrane antigen (EMA) proteins in BCC.
Materials and methodsWe used immunohistochemistry to evaluate the expression pattern of these proteins in 45 BCC specimens and their adjacent normal skin.
ResultsWe found variations in the expression pattern of these proteins among normal skins and BCC. In normal skins, we found strong EMA cytoplasmic expression (adnexal structures). A strong nuclear p63 protein expression was found in basal and some suprabasal keratinocytes, external root sheath cells of the hair follicles, basal cells of the sebaceous glands, and in sweat glands.CD10 protein expression was seen in peri-adnexal mesenchymal spindle cells and myoepithelial cells of sweat glands.BCL-2 protein expression was confined to the basal cell keratinocytes, epidermal melanocytes, outer root sheath, and infundibulum of the hair follicle. In BCC, we found p63 (diffuse, strong nuclear staining), CD10 (focal, moderate cytoplasmic reactivity), and BCL-2 (focal, moderate cytoplasmic reactivity) protein expression in the neoplastic cells. BCC was consistently negative for EMA (except in areas of squamous differentiation).
ConclusionsThere is an altered expression of these proteins in BCC. The underlying molecular mechanisms are open to further investigations.
El carcinoma basocelular (CBC) es una neoplasia cutánea común.
ObjetivoExaminar los patrones de expresión de las proteínas CD10, p63, BCL-2, y antígeno epitelial de la membrana (EMA) en el CBC.
Materiales y métodosUtilizamos inmunohistoquímica para evaluar el patrón de expresión de estas proteínas en 45 muestras de CBC y su piel normal adyacente.
ResultadosEncontramos variaciones del patrón de expresión de estas proteínas entre las pieles normales y el CBC. En las pieles normales, observamos una fuerte expresión citoplasmática de EMA (estructuras anexiales). Se objetivó una fuerte expresión nuclear de la proteína p63 en los queratinocitos basales y algunos suprabasales, células de la vaina de la raíz externa de los folículos pilosos, células basales de las glándulas sebáceas, y glándulas sudoríparas. La expresión de la proteína CD10 se observó en las células fusiformes mesenquimales peri-anexiales y las células mioepiteliales de las glándulas sudoríparas. La expresión de la proteína BCL-2 se confinó en los queratinocitos de las células basales, melanocitos epidérmicos, vaina de la raíz externa, e infundíbulo del folículo piloso. En CBC encontramos expresión de las proteínas p63 (difusa, fuerte tinción nuclear), CD10 (reactividad citoplasmática focal y moderada) y BCL-2 (reactividad citoplasmática focal y moderada) en las células neoplásicas. CBC fue consistentemente negativo para EMA (excepto en zonas de diferenciación escamosa).
ConclusionesExiste una alteración de la expresión de estas proteínas en CBC, quedando abiertos los mecanismos moleculares subyacentes a investigaciones adicionales.
Basal cell carcinoma (BCC) is one of the most common non-melanoma skin cancers worldwide. It commonly occurs in the sun-exposed skin, especially that of the head and neck region. Although BCC is usually an indolent non-aggressive neoplasm that can only locally invade the surrounding tissues, some variants are locally destructively aggressive, and still, others can rarely metastasize (metastatic rate is 0.1%) to the lymph nodes.1,2
The TP63 gene is a member of the p53 gene family, which is located on the chromosome 3q27-29.3 This gene encodes several protein isoforms with both transactivating (TA) and dominant-negative (ΔN) effects on the p53 tumor suppressor gene. The ΔNp63 α isoform can stimulate early steps in tumorigenesis by inhibiting growth arrest and apoptosis, while at the same time it can suppress metastasis by maintaining the epithelial characters of tumor cells.4 At the molecular level, p63 is a nuclear transcription factor that triggers keratinocyte differentiation. In normal skin, p63is strongly expressed by the keratinocytic stem cells, whereas it is down-regulated in terminally differentiated cells.5
Mucins are heavily glycosylated glycoproteins that have protective and lubricating functions. The human MUC1 gene (Mucin-1 gene locus: 1q21) encodes a protein that undergoes glycosylation.6 Its protein product is known as epithelial membrane antigen (EMA), human milk fat globule antigen, polymorphic epithelial mucin, and sialomucin.7 EMA is a 40–425kDa heavily glycosylated protein composed of a transmembrane domain with a cytoplasmic tail and a large extracellular domain that consists of a variable number of tandem repeats which undergoes specific O-glycosylation to generate a broad range of glycosylated variants of the MUC1 – molecule. The integrity of the lumens in the secretory cells depends on the physical and chemical properties (large size, rigidity, and negative charge) of this extracellular domain.6 Some studies indicated the absence of expression of EMA protein expression in BCC which may be reasoned to its altered expression at the level of transcription, aberrant glycosylation, abnormal cellular localization.8–10
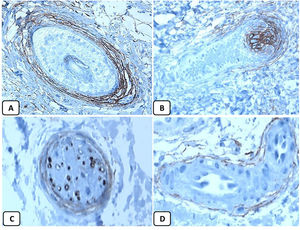
CD10 is a transmembrane glycoprotein recognized as the common acute lymphoblastic leukemia antigen. In the normal skin, CD10 is expressed in the inner sheath of the hair follicles, hair matrix, and perifollicular fibrous sheath. CD10 can integrate signals from the cell environment or the intracellular compartment by cleaving peptides through enzymatic activity or variable intracellular signaling pathways.11 To date, few studies are available about the expression pattern of CD10 protein in the normal skin and BCC.
BCL-2 gene (B-cell lymphoma-2) encodes a protein that prevents cellular apoptosis. In normal skin, BCL-2-positive basal cells act as reserve cells for continuous renewal of the squamous epithelium.12 BCC is believed to arise from these basal cell keratinocytes, and thereforeBCL-2 protein expression is noted in most of these tumors. Alternatively, BCL-2 protein expression is usually negative in squamous cell carcinoma, which has been supposed to arise from the suprabasal keratinocytes.13
The separation of BCC from other cutaneous malignancies and in particular squamous cell carcinoma is important for planning the proper therapeutic management. However, sometimes it is difficult to separate BCC from squamous cell carcinoma of cutaneous origin, especially when the cytoarchitectural features are difficult to assess in small tissue biopsies. Although some previous studies have addressed this issue by reporting the expression pattern of the individual CD10, BCL-2, p63, and EMA proteins in BCC, the results were controversial.8,14,15
To the best of our knowledge, no previous investigations have been performed on the simultaneous comparison of the pattern of expression of these four immunostains together (including p63, EMA, CD10, and BCL-2) either in BCC or in its adjacent normal skin. Moreover, correlations among the expression of these proteins and clinicopathologic features of BCC and their pathogenetic roles in the development of BCC were not previously addressed. We carried this investigation to accomplish these goals. Our study demonstrates variations in the distribution of these proteins among normal skin and BCC. The diagnostic and pathogenetic aspects of our findings are presented.
Materials and methodsPatientsThis retrospective study was carried out at the Faculty of Medicine after Department of Pathology, Assiut University Hospitals. The study was approved by the Ethical Committee of the Faculty of Medicine, Assiut University (IRB #: 17300540). A total of 45 cases of BCC were included, and their full clinicopathological data were obtained from the Pathology reports (consultation files of the authors). Full pathologic features were recorded. All the materials (paraffin-embedded tissue blocks, slides, and pathology reports) were coded. The information obtained was analyzed and reported in such a way that the identities of the participants cannot be ascertained. The study did not include any interaction or intervention with human subjects or included any access to any identifiable private information.
ImmunohistochemistryThe presence of p63, CD10, BCL-2, and EMA proteins was examined using Avidin-biotin immunoperoxidase complex following other groups.16 Briefly, 4-μm thick tissue sections mounted on glass slides were deparaffinized and rehydrated through graded alcohols to water. Endogenous peroxidase was blocked with 3% hydrogen peroxide in methanol for 5min. Sections were then immersed in the retrieval solution (10mM sodium citrate buffer, pH 7.3) and subjected to heat-induced antigen retrieval for 10min. The slides, in plastic Coplin jars containing retrieval solution, were microwaved in a microwave set at high (∼700W). Non-specific protein binding was blocked with 10min of exposure to 10% normal goat serum. Sections were then incubated with monoclonal antibodies raised against p63, CD10, BCL-2, and EMA (Agilent/DAKO, 5301 Stevens Creek Blvd, Santa Clara, CA 95051, United States) for 30min at room temperature (Clone 56C6 for CD10, Clone: DAK-p63 for p63, Clone: E29 for EMA, and Clone: 124 for BCL-2). A detection system was used according to the manufacturer's instructions. Sections were next treated with peroxidase-labeled streptavidin for 30min at room temperature and incubated with 14-diaminobenzidine and 0.06% H2O2 for 5min. They were counterstained with Mayer hematoxylin, dehydrated in alcohol, cleared in xylene, and cover-slipped. The staining was independently evaluated by the two authors.
Positive controlSections from the tonsils (BCL-2), prostate (p63), and kidney (EMA and CD10), were included as positive controls. The positivity for EMA was identified as brownish cytoplasmic and membranous staining. The positivity for p63 was identified as brownish staining of the nuclei. The positivity for CD10 and BCL-2 was identified as brownish cytoplasmic staining.
Negative controlAdditional sections were stained in parallel with the omission of the primary antibodies as a negative control. The positive and negative controls were positive and negative, respectively, indicating the validity of our results. All slides were evaluated without knowing the patient's information and clinical reports.
Statistical analysisThe statistical software package SPSS version 16 was used for all analyses. The Chi-square and Fisher's exact tests were used as appropriate. The p-value of less than 0.05 was regarded as statistically significant.
ResultsThis retrospective study included 45 patients with BCC; of them, there were 26 females and 19 males with a mean age of 65.5±11.25 years (mean±SD). Most BCCs were in the head and neck region, except for a few cases that occurred on the extremities and back. The histologic variants included nodular, micronodular, pigmented, adenoid, basosquamous, and morpheaform patterns. The clinic-pathologic features are summarized in Table 1.
Clinicopathological features of basal cell carcinomas.
| Aspects | Total number of case=45 |
|---|---|
| Sex | |
| Male | 19 (42.2%) |
| Female | 26 (57.8%) |
| Site | |
| Head and neck region | 30 (66.7%) |
| Left lateral ala skin | 3 |
| Ear | 3 |
| Eyelid | 2 |
| Forehead | 4 |
| Hairline | 3 |
| Left mid helix | 1 |
| Lip | 3 |
| Neck | 4 |
| Nose | 1 |
| Peri-auricular | 3 |
| Scalp | 3 |
| Extremities and truck | 15 (33.3%) |
| Shoulder | 4 |
| Arm | 3 |
| Back | 3 |
| Clavicle | 2 |
| Forearm | 2 |
| Lumbar region | 1 |
| Gross appearance | |
| Ulcerated lesion | 35 (77.8%) |
| Non-ulcerated nodule | 10 (22.2%) |
| Histologic variants | |
| Nodular BCC | 15 (33.3%) |
| Micronodular | 7 (15.6%) |
| Pigmented BCC | 6 (13.3%) |
| Adenoid BCC | 4 (8.9%) |
| Morpheaform pattern | 2 (4.5%) |
| Baso-squamous | 11 (24.4) |
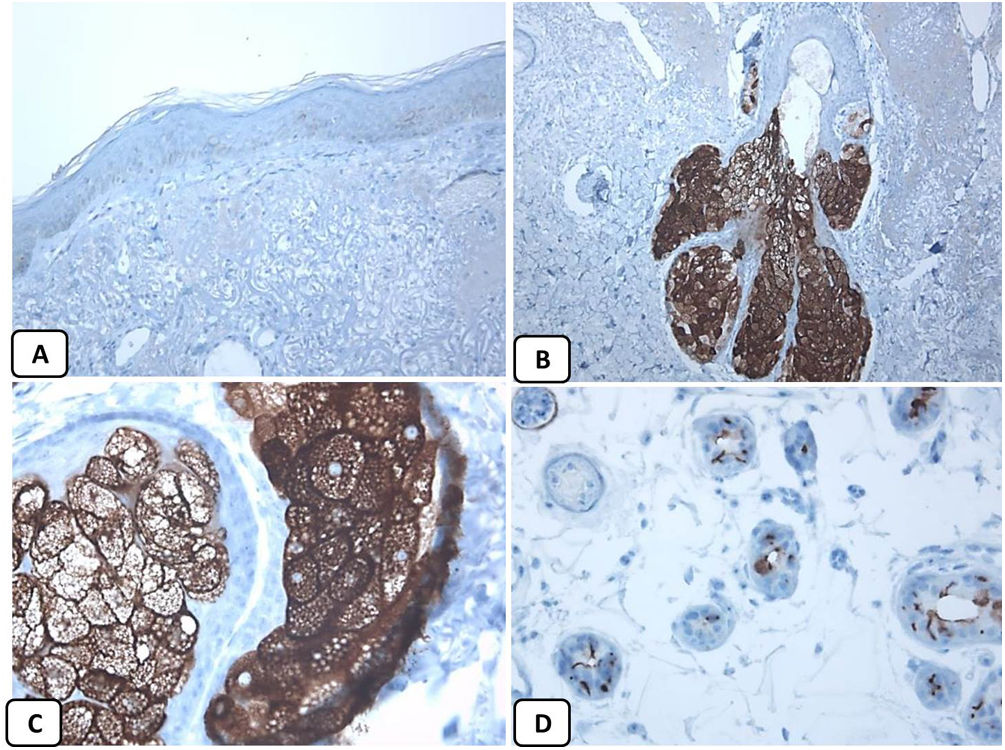
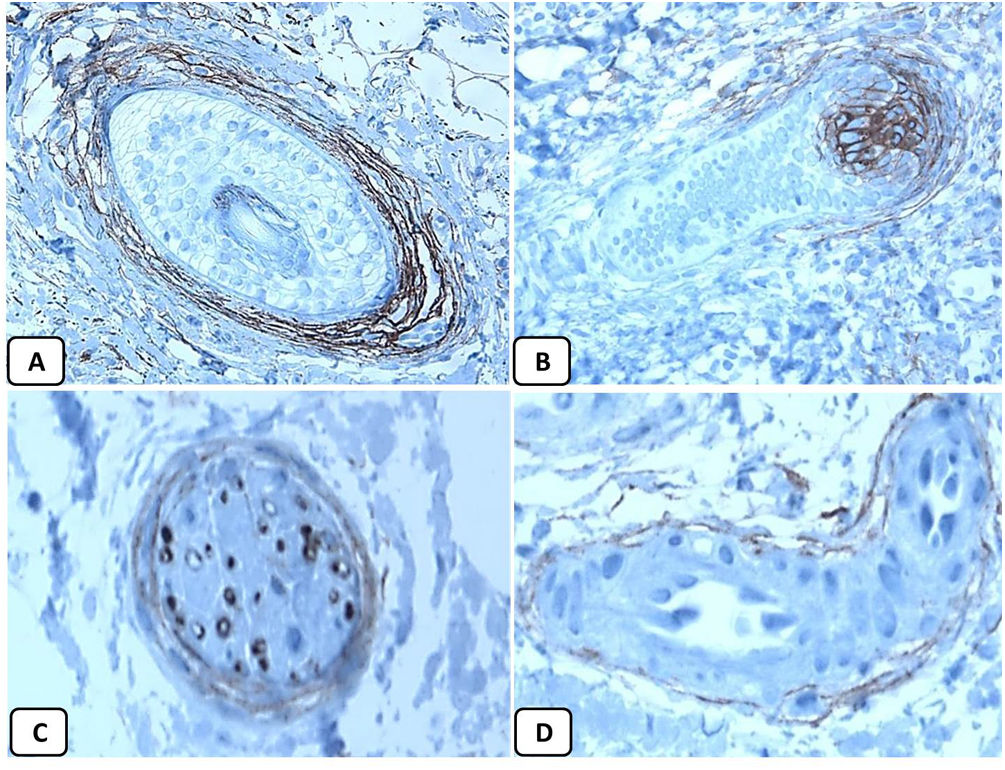
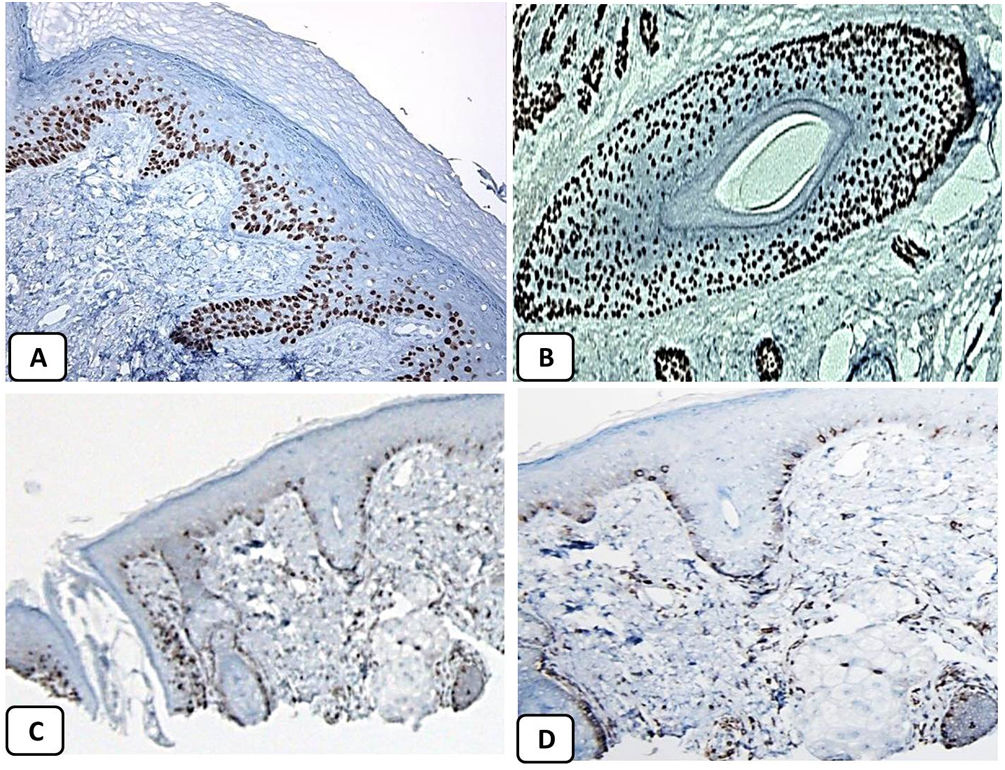
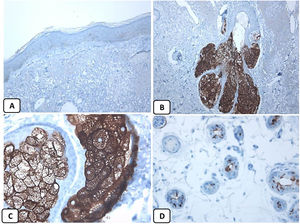
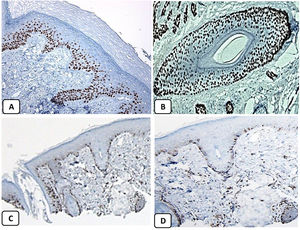
The positive and negative controls were positive and negative, respectively, indicating the validity of our results. p63 protein nuclear positivity was detected in the basal and some suprabasal epidermal keratinocytes, cells of the external root sheath of the hair follicles, and in basal cells of the sebaceous glands and sweat glands. EMA showed strong expression in the cytoplasm of the sebaceous glands, the luminal membranes, the canaliculi of the sweat glands, and the outer layer of sweat duct cells, whereas the protein expression was absent in the epidermis. CD10 protein expression was detected in peri-adnexal mesenchymal spindle cells surrounding dermal appendages, some nerve axons, hair papilla of vellus follicles, and myoepithelial cells of the sweat glands. BCL-2 protein expression was confined to the epidermal basal keratinocytes and melanocytes. BCL-2 protein expression was also observed in the keratinized outer root sheath of the isthmus and infundibulum of the hair follicle. A summary of these findings is shown in Figs. 1–3.
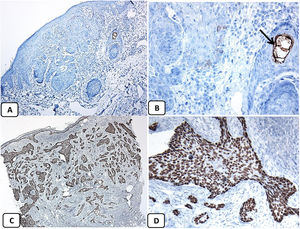
Immunohistochemical expression of EMA in the normal skin. Negative EMA staining in the epidermis (A, ×200), strong EMA expression in the cytoplasm of mature sebaceous glands (B, ×200 and C, ×400). D: positive EMA in the luminal membrane and canaliculi of sweat gland and the outer layer of sweat duct (D, ×200).
Immunohistochemical expression of p63, and BCL 2 in the normal skin. p63 nuclear positivity in the basal and some suprabasal epidermal cells (A, ×400) and cells of the external root sheath of the hair follicles (B, ×400). Positive BCL-2 expression in the basal keratinocytes in the epidermis and the keratinized outer root sheath of the isthmus and infundibulum of the hair follicle (C, ×200 and D, ×400).
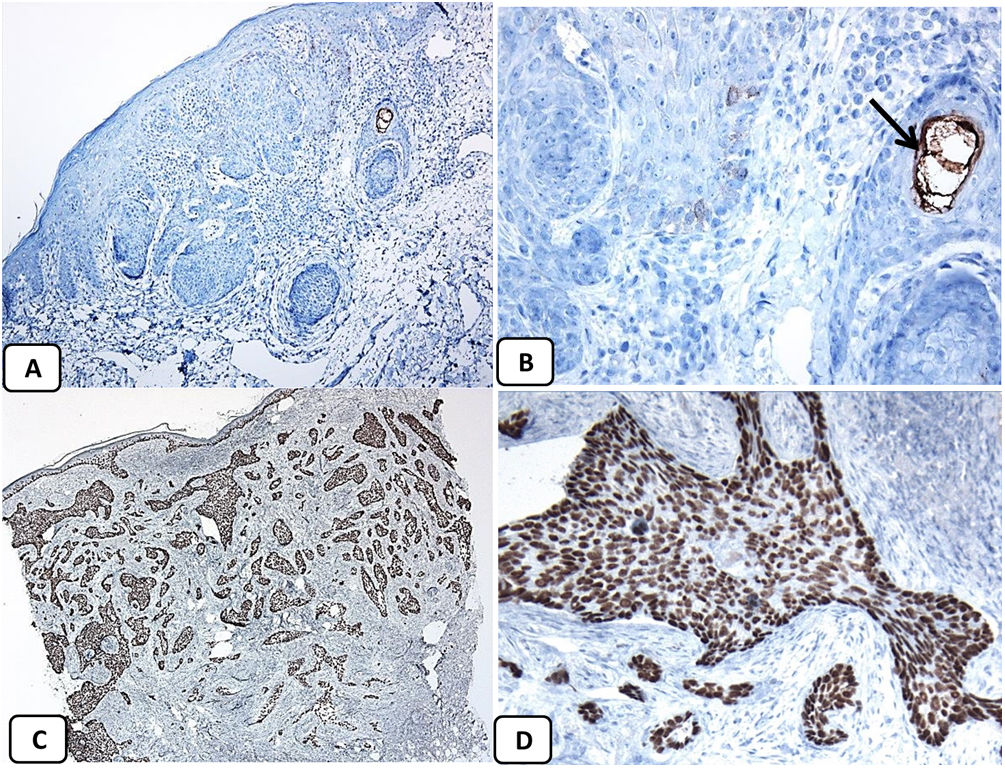
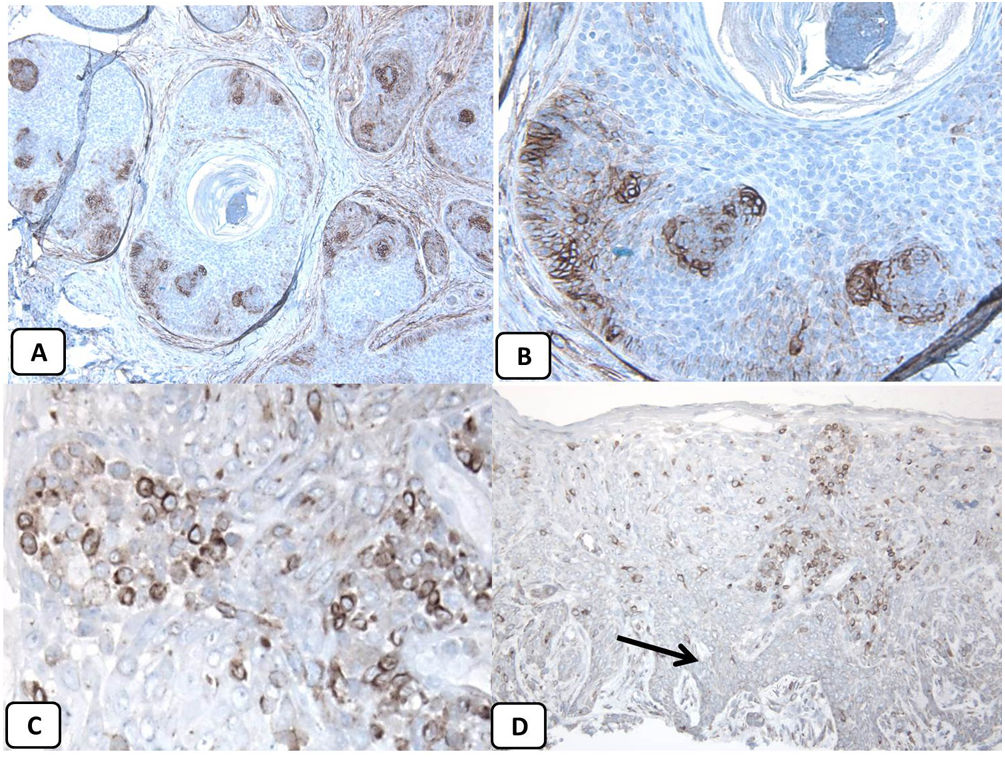
p63 protein was seen in all cases of BCC and showed consistently diffusely and strongly positive (nuclear staining) in the tumor cells of all histological subtypes. EMA protein expression was absent in all BCC specimens except for areas of squamous differentiation. CD10 protein expression was seen in 31 BCC specimens (68.9% of cases), with more reactivity in the peripheral cells. Focal weak to moderate staining of BCL2 was seen in all cases of BCC, especially among the peripheral cells. BCL-2 protein expression was reduced in the infiltrative areas of BCC.
As all cases were positive for p63, BCL-2, and negative for EMA while 68.9% were positive for CD10, the association between clinicopathologic features and these proteins expression could be statistically performed as regard CD10 only. There were no significant associations between CD10 expression and the clinicopathologic parameters of BCC (Table 2). A summary of these findings is shown in Figs. 4 and 5.
The association between the CD10 expression and the clinicopathological features of basal cell carcinomas.
| Clinicopathologic feature | CD10 expression | ||
|---|---|---|---|
| + | − | p value | |
| (%) | (%) | ||
| Sex | |||
| Male | 15 (78.9) | 4 (21.1) | 0.213 |
| Female | 16 (61.5) | 10 (38.5) | |
| Site | |||
| Head and neck region | 19 (63.3) | 11 (36.7) | 0.255 |
| Extremities and truck | 12 (80) | 3 (20) | |
| Gross appearance | |||
| Ulcerated lesion | 24 (68.6) | 11 (31.4) | 0.931 |
| Non-ulcerated nodule | 7 (70) | 3 (30) | |
| Histologic variants | |||
| Nodular BCC | 11 (73.3) | 4 (26.7) | 0.442 |
| Pigmented BCC | 5 (83.3) | 1 (16.7) | |
| Micronodular BCC | 3 (42.9) | 4 (57.1) | |
| Basosquamous | 9 (81.8) | 2 (18.2) | |
| Morpheaform | 1 (50) | 1 (50) | |
| Adenoid BCC | 2 (50) | 2 (50) | |
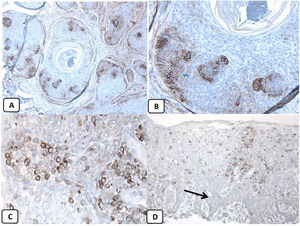
Immunohistochemical expression of CD10, and BCL 2 proteins in basal cell carcinoma. (A and B) CD10 cytoplasmic staining in BCC cells with more positivity in the peripheral cells (A, ×100 and B, ×400). (C and D) Positive cytoplasmic BCL2 expression in BCC especially among the peripheral cells (C, ×400). Reduced BCL2 expression in the infiltrative areas (arrow) is noted (D, ×200).
Here we report the expression patterns of p63, EMA, CD10, and BCL-2 proteins in the normal skin and BCC. Our study demonstrates the following: (i) there is no correlation between the expression of these proteins and the clinicopathologic features, and (ii) variations in the expression pattern between the normal skin and BCC.
Strong nuclear p63 protein expression in BCCIn line with previous studies,5,17,18 we found diffuse and strongnuclearp63 protein expression in BCC whereas, in the normal skin, p63 reactivity was only seen in the basal and suprabasal keratinocytes. The nuclear expression of p63 in BCC helps separate the pigmented variants of these tumors from malignant melanomas. The strong diffuse expression of p63 in the progenitor/basaloid cells of the normal epidermis as well as in the neoplastic cells of BCC versus its absence in the terminally differentiated squamous cells suggests that p63 acts as a keratinocyte stem cell protein. The p63 protein expression in BCC not only indicates that neoplastic cells are derived from basaloid progenitor cells of squamous lineage but also suggests its role in tumorigenesis of this neoplasm.5
To date, the role of p63 in the development of BCC is largely unknown. The p63 gene encodes six proteins with both transactivating (TA) and dominant-negative (ΔN) effects on the p53 gene. The p53 protein promotes apoptosis and induces cell cycle arrest. The ΔN-p63α isoforms play their oncogenic functions by inhibiting p53-mediated cell cycle arrest and apoptosis in the basal cells.17,19 The dominant-negative ΔN-p63α isoform can mediate oncogenesis through different mechanisms, including activation of β-catenin pathway,20 suppression of the proapoptotic protein insulin-like growth factor binding protein-3,20 up-regulation of Hsp-70,21 and repression of p73-dependent apoptosis.22 The endogenous p63 can suppress genes involved in tumor metastasis, which explains the association between the loss of p63 and bad prognosis in cancers.4 The loss of the p63 gene seems to result in the up-regulation of genes involved in the invasion and metastasis as N-cadherin.4 In support, Johnson et al. reported the expression of p63 protein in the primary cutaneous BCC versus its loss in BCC metastatic to the lymph nodes.23 Taken as a whole, we propose that the development of BCC may be related to the expression of p63 through its dominant-negative effects on the tumor suppressor p53. Moreover, the lack of metastatic potential in BCC may be related to the strong p63 protein expression in these tumors. Interestingly, p63 can interact with the sonic hedgehog signaling pathway that is commonly involved in the pathogenesis of BCC.24
Absence of EMA protein expression in BCCWe found variations in the expression pattern of EMA between the normal skin and BCC. The expression of EMA in the normal skin in our series concurs with previous studies. Modrzynsk et al. reported EMA expression in the eccrine, sebaceous, and apocrine glands of the normal skin. The normal squamous epithelium is EMA negative, except for that covering the tonsils.25 In agreement with other studies, we found the absence of EMA protein expression in BCC, except focally in the areas with squamous differentiation.8,15,26 Alternatively, previous studies indicated the consistent expression of EMA protein in squamous cell carcinoma.10,27 Taken together, these findings emphasize the diagnostic value of EMA in separating BCC from squamous cell carcinoma.
Although the underlying molecular mechanisms for EMA protein expression in the areas with squamous differentiation within BCC are mostly unknown, it may be reasoned to EMA protein mislocalization, aberrant glycosylation, or altered transcriptional regulation. In these squamous areas, EMA proteins may be depolarized (mislocalization), i.e., the protein expression is no longer restricted to the apical domain, but instead, they are expressed over the entire cell surface as well as in the cytoplasm. This depolarized expression enables interactions between membrane-bound EMA and other cellular proteins such as the human epidermal receptor family of receptor tyrosine kinases, with subsequent activation of signaling pathways resulting in cell proliferation and survival.6,28 It is still possible that the aberrant expression of EMA protein in the squamous areas is due to its aberrant glycosylation (hypoglycosylation and different carbohydrate composition) inside the squamous cells. This hypoglycosylation results in exposing the core protein of the tandem repeat region.6
Expression of CD10 in BCCIn agreement with the previous studies, we observed variable expression of CD10 in the majority of BCC, with focal weak to moderate peripheral CD10 staining in the neoplastic cells of BCC.8,29–32 Shamsi Meymandi et al. used immunohistochemical techniques to examine the expression of CD10, CD1a, SMA, Ki67, CD34, and P53 proteins in both BCC1 or low risk (nodular, superficial type) and BCC2 or high risk (micronodular, morpheaform, infiltrative, and basosquamous types) tumors. The counts of the epidermal Langerhans cells above the tumor are higher in BCC1 as compared to BCC2. The percentage of p53-positive tumor cells was high in BCC2 when compared to BCC1. The mean counts of blood vessels (CD34) were low in BCC1 as compared to BCC2.32
Wagoner et al. evaluated CD 10 expression in BCC, and they found strong CD10 protein expression in the superficial BCC versus the loss of expression in the deeply infiltrative parts of the tumors.29 The authors suggested that strong CD10 expression in the tumor cells of the superficial BCC is related to its indolent nature, suggesting a possible role in the development of these tumors.29 Alternatively, previous studies indicated a lack of expression of CD10 protein in squamous cell carcinoma.13
To date, the underlying mechanisms for this variable expression of CD10 in BCC are mostly unknown. In carcinogenesis, CD10 behaves as a double edge sword, i.e., a dual rule in cancer depending on the peptides present in the surrounding tumor microenvironment that modulate cancer progression.33 In support, high expression ofCD10 was associated with cancer progression and migration in esophageal squamous carcinoma.11,34 This role in tumor progression is related to the ability of CD10 to create a tumor microenvironment that facilitates tumor cell migration and metastasis due to its structural similarity to matrix metalloproteinases.35 Also, decreased CD10 expression in cervical squamous carcinoma was associated with increased cancer progression.36 This decreased expression is associated with increased peptide concentrations in the tumor microenvironment, which can facilitate intracellular signaling and tumor proliferation.33 Alternatively, CD10 can also behave as a tumor suppressor in some tumors by inhibiting several events involved in tumor progression. Taken together, we propose that the weak expression of CD10 in the deep portions of BCC is related to the increased aggressiveness of these tumors.
BCL-2 protein expression in BCCWe found BCL 2 protein expression in the basal layer of the epidermis, papillae of hair follicles, and the follicular bulges. Our findings agree with the previous studies37,38 and suggest a role for BCL2 in the homeostasis of the skin. In agreement with other studies, our series revealed focal weak to moderate BCL2 protein expression in BCC, especially among the peripheral cells, while the expression was reduced in the infiltrative areas.14,39–41 BCL2 protein expression in BCC supports the notion that these tumors are derived from the basal cells of the epidermis or the stem cells located in the bulge of the hair follicle.42 Crowson et al. reported different BCL2 protein expressions in the indolent and aggressive BCC subtypes and suggested that BCL2 may play an essential role in the pathogenesis and biological behavior of BCC.39 These authors proposed that BCL2 protein overexpression may lead to the accumulation of the primitive basally derived neoplastic cells and the development of the non-aggressive circumscribed and superficial BCC. The prolonged life span of these cells resulted in excessive exposure to ultraviolet light and the acquisition of additional genetic alterations that transform indolent BCC into aggressive form.39
Deng et al. addressed the molecular changes (BCL-2 and Ki67) in BCC. The authors indicated a relationship between these changes and the clinical and prognostic features of BCC. Some gross characteristics (such as friability) and histological features (tumor retraction) are related to cell–cell adhesion and basement membrane characteristics of these tumors.41
Zhang et al. examined the immunohistochemical features of trichoblastoma (58 cases) and BCC (40 cases). They used bcl-2, Ber-EP4, CD10, CK20 and Ki-67 immunostains. CK20 was negative in BCC but positive in most cases of trichoblastoma. The proportion of BCC with BCL-2 reactivity was higher than that of trichoblastomas. The proportion of cells with Ber-EP4 reactivity was higher in BCC than in trichoblastomas. CD10 protein expression was seen in the follicular stroma of the majority of trichoblastoma whereas the expression was focal in the stroma of BCC.31
To conclude, here we addressed the expression patterns of CD10, BCL-2, p63, and EMA in the normal skin and BCC. EMA seems to be the most reliable marker for separating BCC from squamous cell carcinoma. Moreover, the variations in the expression pattern between the normal skin and BCC suggest possible pathogenetic roles for these proteins in the development of these neoplasms. The underlying molecular mechanisms are open for further investigations.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations Ethics approval and consent to participateObtained (Pathology Department/Faculty of Medicine, Assiut University).
Competing interestsThe authors declare that they have no competing interests.
Authors’ contributionsMH conceived the idea and generated the data, AA analyzed and interpreted the patient data. MH and AA performed the histological analysis, and both MH and AA contributed equally in writing the manuscript. All authors read and approved the final manuscript.