Cutaneous squamous cell carcinoma (CSCC) metastatic to lymph nodes is an entity with low incidence (2.4% in males and 1.1% in females), which often poses a therapeutic challenge. Traditionally, systemic treatment of nonresectable cases has involved platinum-based antineoplastic drugs and epidermal growth-factor inhibitors, with or without radiation therapy. Recent studies, however, increasingly point to immune checkpoint inhibitors as the most effective and safe alternative for treatment of locally advanced or metastatic disease. We report the case of a patient with CSCC metastatic to the lymph nodes, who showed a complete objective response after 6 months of treatment with pembrolizumab.
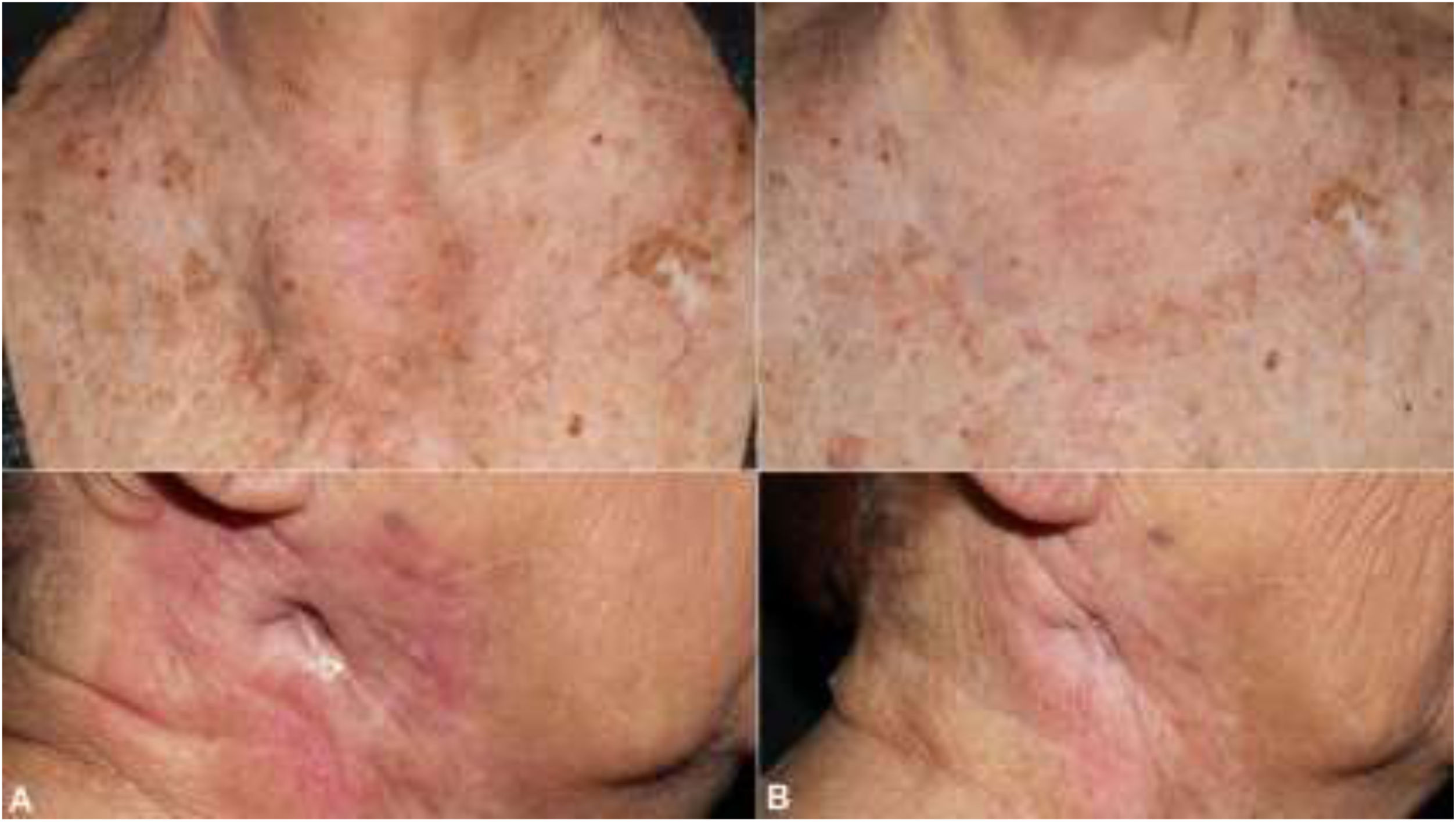
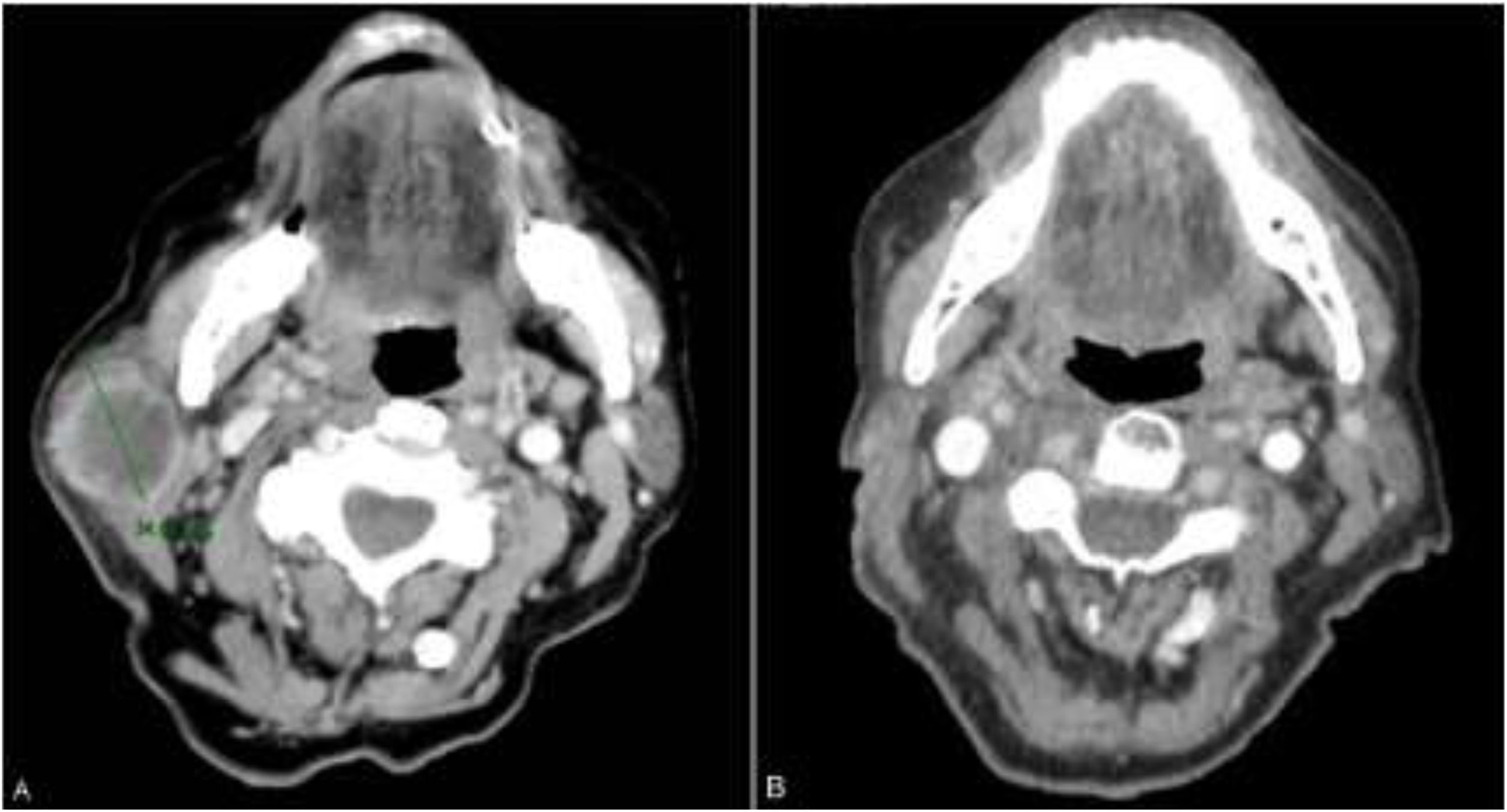
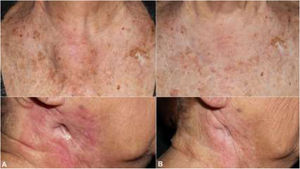
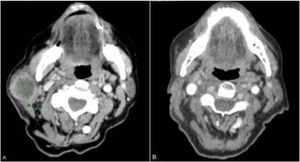
The patient was an 83-year-old woman who was treated for moderately differentiated and infiltrating CSCC with a thickness of 8 mm, with no lymph-vessel or perineural involvement and spared margins, in the right mandibular ramus. Expression of programmed cell death ligand (PD-L1) was 30% in cancerous cells. The patient’s personal history included a diagnosis of invasive ductal carcinoma that did not progress and was not treated. Three months after the intervention, the patient developed a metastatic conglomerate lymph-node mass in the angle of the right side of the jaw, measuring 4.5 cm; the mass was confirmed to be squamous cell carcinoma by means of fine-needle aspiration cytology. Curative radiation therapy was performed but the lymph-node disease progressed and an anterior cervical mass measuring up to 8 cm developed. In light of the nonresectable nature of the disease and its progression despite radiation therapy, with an ECOG score of 0, permission was sought for off-label use of pembrolizumab. The patient began treatment with pembrolizumab at a dosage of 2 mg/kg every 3 weeks, with rapid reduction in tumor size after 4 cycles and complete clinical and radiologic remission maintained over 6 (Figs. 1 and 2). The treatment was well tolerated, and the patient presented only a syndrome similar to rheumatic polymyalgia, which remitted with analgesics and 200 mg of hydroxychloroquine every 12 hours. At the same time, the ductal carcinoma showed no alternations in the control mammograms.
The role of immunotherapy in skin cancer is becoming increasingly important. PD-1 inhibitors are producing highly promising results for the treatment of locally advanced and metastatic CSCC,1–5 with a response rate of up to 60% according to the latest reviews - mostly in the form of partial responses.4 Cemiplimab, a programmed cell death receptor 1 (PD-1) antibody, has recently been approved with indications by the EMA and the FDA for locally advanced CSCC. Pembrolizumab is an anti-PD-1 monoclonal antibody indicated as adjuvant treatment in resected stage iii melanoma, advanced melanoma, Hodgkin lymphoma, urothelial carcinoma, non-small cell lung cancer, and squamous cell carcinoma of the head and neck. Its mechanism of action affects the immunological synapse, inhibiting the coinhibitory activity of PD-1, thus favoring destruction of the tumor by intratumoral CD8 T cells.6 Studies exist on the expression of PD-1 and PD-L1, and on the type of intratumoral inflammatory infiltrate and its relation to tumor characteristics7 and treatment response.8
It is not clear that a cutoff exists in the expression of PD-1 and PD-L1 in CSCC tumor cells and its relation to the response to anti-PD-1 drugs, although a positive correlation appears to exist.7,8 Similarly, expression of PD-L1 has also been linked to high-risk characteristics such as the infiltration pattern, perineural invasion, and immunosuppression.7 To date, most publish results are of partial responses,4 although complete responses have also been reported (Table1); the time for which treatment should be maintained is therefore not clear.1–3 Nevertheless, this is a treatment with a good safety profile, which is especially important given that it is used mostly in elderly patients.4,9 Immune-mediated adverse effects may appear during treatment or even months after treatment has been suspended. The most frequent of these are cutaneous adverse effects, followed by colitis, hepatitis, endocrinologic effects, pneumonitis, and arthritis.10 In general, immune-mediated adverse effects tend to be mild (grade 1–2), and can be managed in an outpatient setting with oral corticosteroid dosages of 0.5–1 mg/kg/d of prednisone. More severe cases (grade 3) require admission to hospital, temporary suspension of the immunotherapy, and treatment with intravenous systemic corticosteroids at dosages of between 1 and 2 mg/kg/d; immunosuppressive therapy should be considered if no response is achieved after 48–72 hours.10 We report a case of locally advanced CSCC in an elderly patient, with complete response following treatment with pembrolizumab as first-line systemic treatment.
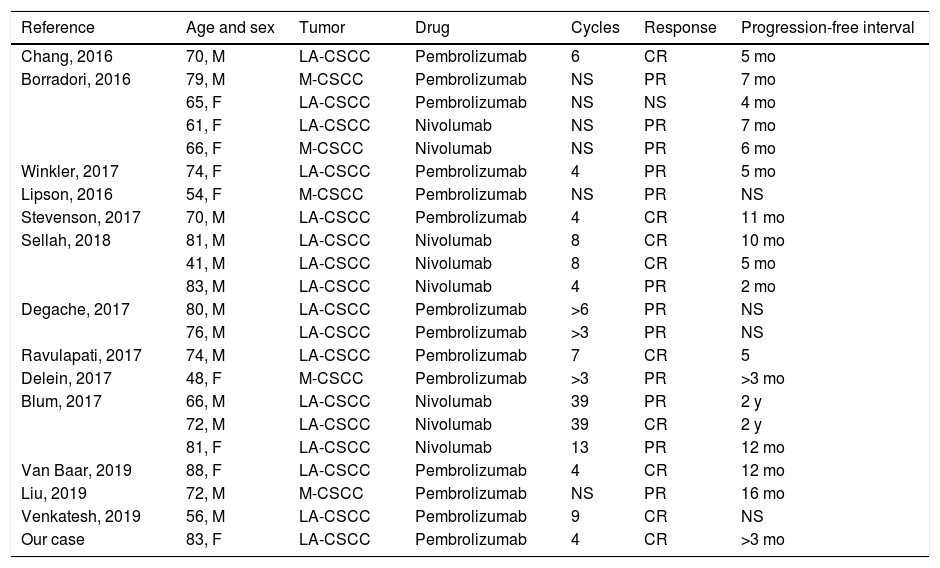
Review of cases of advanced squamous cell carcinoma treated with anti-PD1 in the literature.
| Reference | Age and sex | Tumor | Drug | Cycles | Response | Progression-free interval |
|---|---|---|---|---|---|---|
| Chang, 2016 | 70, M | LA-CSCC | Pembrolizumab | 6 | CR | 5 mo |
| Borradori, 2016 | 79, M | M-CSCC | Pembrolizumab | NS | PR | 7 mo |
| 65, F | LA-CSCC | Pembrolizumab | NS | NS | 4 mo | |
| 61, F | LA-CSCC | Nivolumab | NS | PR | 7 mo | |
| 66, F | M-CSCC | Nivolumab | NS | PR | 6 mo | |
| Winkler, 2017 | 74, F | LA-CSCC | Pembrolizumab | 4 | PR | 5 mo |
| Lipson, 2016 | 54, F | M-CSCC | Pembrolizumab | NS | PR | NS |
| Stevenson, 2017 | 70, M | LA-CSCC | Pembrolizumab | 4 | CR | 11 mo |
| Sellah, 2018 | 81, M | LA-CSCC | Nivolumab | 8 | CR | 10 mo |
| 41, M | LA-CSCC | Nivolumab | 8 | CR | 5 mo | |
| 83, M | LA-CSCC | Nivolumab | 4 | PR | 2 mo | |
| Degache, 2017 | 80, M | LA-CSCC | Pembrolizumab | >6 | PR | NS |
| 76, M | LA-CSCC | Pembrolizumab | >3 | PR | NS | |
| Ravulapati, 2017 | 74, M | LA-CSCC | Pembrolizumab | 7 | CR | 5 |
| Delein, 2017 | 48, F | M-CSCC | Pembrolizumab | >3 | PR | >3 mo |
| Blum, 2017 | 66, M | LA-CSCC | Nivolumab | 39 | PR | 2 y |
| 72, M | LA-CSCC | Nivolumab | 39 | CR | 2 y | |
| 81, F | LA-CSCC | Nivolumab | 13 | PR | 12 mo | |
| Van Baar, 2019 | 88, F | LA-CSCC | Pembrolizumab | 4 | CR | 12 mo |
| Liu, 2019 | 72, M | M-CSCC | Pembrolizumab | NS | PR | 16 mo |
| Venkatesh, 2019 | 56, M | LA-CSCC | Pembrolizumab | 9 | CR | NS |
| Our case | 83, F | LA-CSCC | Pembrolizumab | 4 | CR | >3 mo |
Abbreviations: LA-CSCC indicates locally advanced cutaneous squamous cell carcinoma; M-CSCC, metastatic cutaneous squamous cell carcinoma; M, male; NS, not specified; CR, complete response; PR, partial response.
The authors declare that they have no conflicts of interest.
Please cite this article as: Villegas-Romero I, Jiménez-Gallo D, Gutiérrez-Bayard L, Linares-Barrios M. Carcinoma epidermoide cutáneo avanzado tratado con pembrolizumab. Actas Dermosifiliogr. 2021;112:672–675.