Oral roflumilast is a phosphodiesterase-4 inhibitor approved for the prevention of exacerbations of chronic obstructive pulmonary disease and chronic bronchitis. In dermatology, topical roflumilast is authorized by the US Food and Drug Administration for the treatment of plaque psoriasis and mild to moderate seborrheic dermatitis. Several studies have described the off-label use of roflumilast in dermatology, including a randomized controlled trial showing its usefulness in the treatment of psoriasis; case reports and small series have also reported successful outcomes in hidradenitis suppurativa, recurrent oral aphthosis, nummular eczema, lichen planus, and Behçet disease. Roflumilast has a favorable safety profile, similar to that of apremilast, and it is considerably cheaper than new generation drugs and even some conventional immunosuppressants. We review the pharmacokinetics and pharmacodynamics of topical and oral roflumilast and discuss potential adverse effects and both approved and off-label uses in dermatology. Roflumilast is a promising agent to consider.
Roflumilast es un inhibidor de la fosfodiesterasa-4 aprobado de forma oral para la prevención de exacerbaciones en pacientes con enfermedad pulmonar obstructiva crónica y fenotipo de bronquitis crónica. En dermatología, el roflumilast tópico está aprobado por la Food and Drug Administration en psoriasis en placas y dermatitis seborreica leve/moderada. En cuanto a su uso fuera de indicación, hemos encontrado un ensayo clínico que avala la utilidad del roflumilast oral en psoriasis, así como pequeñas series de casos o casos clínicos aislados en hidradenitis supurativa, aftosis oral recurrente, eccema numular, liquen plano y enfermedad de Behçet. Su perfil de seguridad es favorable, similar al del apremilast, y su coste es considerablemente inferior a los de los fármacos de nueva generación, o incluso al de algunos inmunosupresores clásicos. Presentamos una revisión de roflumilast tópico y oral, en términos de farmacocinética y farmacodinámica, efectos adversos, usos dermatológicos aprobados y fuera de indicación. Roflumilast es un agente prometedor en dermatología.
The arrival of biologic drugs and small molecules has revolutionized the management of multiple inflammatory dermatoses such as psoriasis, atopic dermatitis, hidradenitis suppurativa, and alopecia areata, among others. However, their high cost poses a significant limitation, especially when used off-label.1
Apremilast is an oral inhibitor of the enzyme phosphodiesterase-4 (PDE4), with immunomodulatory effects and no immunosuppression. It has been approved by the U.S. Food and Drug Administration (FDA) for the management of psoriasis,2 psoriatic arthritis, and oral ulcers in patients with Behçet's disease.3 Its safety profile is just excellent and has been successfully used off-label to treat multiple dermatoses.3 Crisaborole is a topical PDE4 inhibitor approved to treat atopic dermatitis.4 Roflumilast is another PDE4 inhibitor (PDE4i). Its oral form was initially approved by the European Medicines Agency (EMA) and the FDA to reduce the risk of exacerbations in chronic obstructive pulmonary disease (COPD) and chronic bronchitis phenotype.5 Topical roflumilast cream at 0.3% was approved by the FDA in 2022 to treat of plaque psoriasis in patients older than 12 years, making it the first topical PDE4 inhibitor to be approved for the management of plaque psoriasis.6 Back in April 2023, the FDA approved its topical use at 0.3% to treat seborrheic dermatitis.7 Thanks to its excellent safety profile, ease of dosing, and low cost (the price of oral roflumilast is around €35/month in Spain), this drug has been used orally to treat plaque psoriasis, recurrent oral aphthosis, hidradenitis suppurativa, nummular eczema, and lichen planus, among other dermatoses. Its potential uses are not only limited to the dermatology setting, but also extend to cognitive impairment, dementia, schizophrenia, and other neurological or psychiatric conditions,8–13 ulcerative colitis, diabetes mellitus, obesity, polycystic ovary syndrome, asthma, bronchiectasis, and cystic fibrosis14–19 (table 1). In this article, we’ll go over the mechanism of action, pharmacokinetics, long-term safety profile, and especially off-label uses of roflumilast in dermatology. We’ll also discuss diseases that could potentially benefit from this drug due to its similar mechanism of action compred to apremilast.
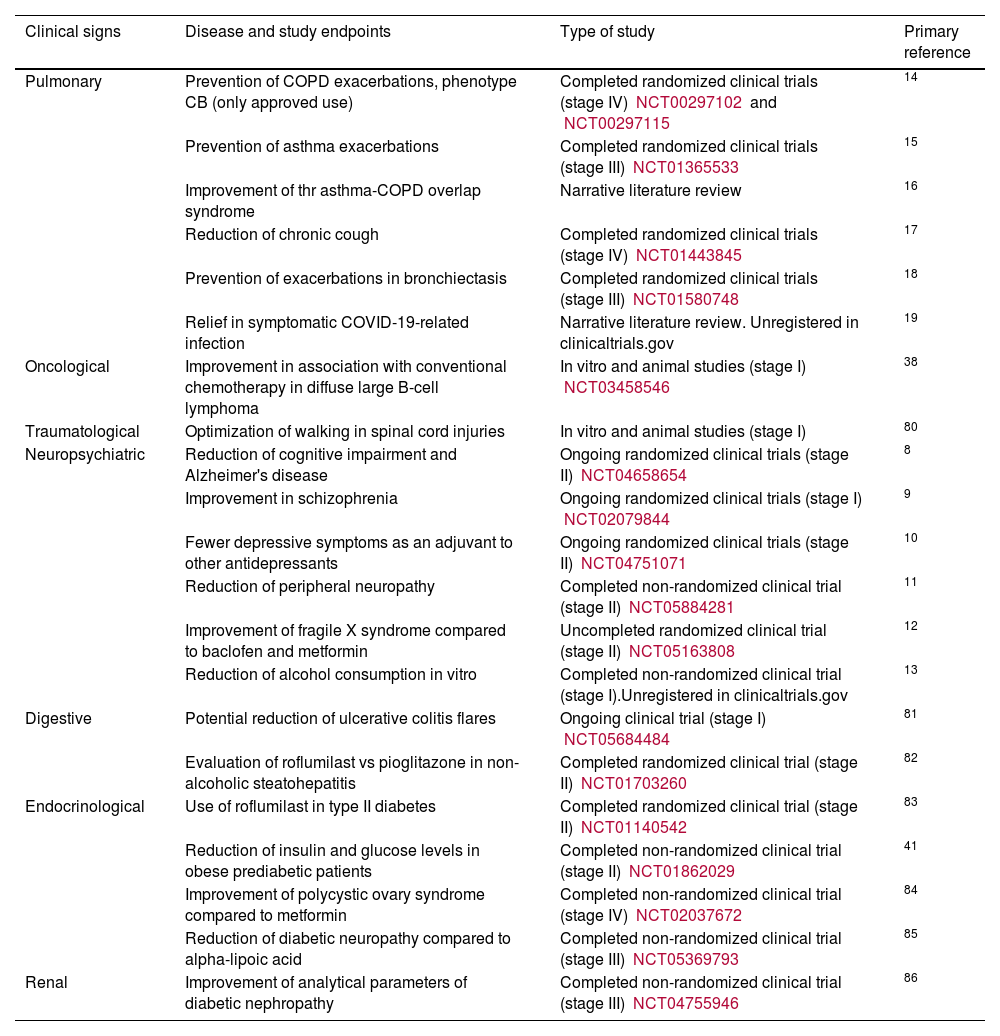
Uses of roflumilast in non-dermatological diseases.8,38,80-86
| Clinical signs | Disease and study endpoints | Type of study | Primary reference |
|---|---|---|---|
| Pulmonary | Prevention of COPD exacerbations, phenotype CB (only approved use) | Completed randomized clinical trials (stage IV) NCT00297102 and NCT00297115 | 14 |
| Prevention of asthma exacerbations | Completed randomized clinical trials (stage III) NCT01365533 | 15 | |
| Improvement of thr asthma-COPD overlap syndrome | Narrative literature review | 16 | |
| Reduction of chronic cough | Completed randomized clinical trials (stage IV) NCT01443845 | 17 | |
| Prevention of exacerbations in bronchiectasis | Completed randomized clinical trials (stage III) NCT01580748 | 18 | |
| Relief in symptomatic COVID-19-related infection | Narrative literature review. Unregistered in clinicaltrials.gov | 19 | |
| Oncological | Improvement in association with conventional chemotherapy in diffuse large B-cell lymphoma | In vitro and animal studies (stage I) NCT03458546 | 38 |
| Traumatological | Optimization of walking in spinal cord injuries | In vitro and animal studies (stage I) | 80 |
| Neuropsychiatric | Reduction of cognitive impairment and Alzheimer's disease | Ongoing randomized clinical trials (stage II) NCT04658654 | 8 |
| Improvement in schizophrenia | Ongoing randomized clinical trials (stage I) NCT02079844 | 9 | |
| Fewer depressive symptoms as an adjuvant to other antidepressants | Ongoing randomized clinical trials (stage II) NCT04751071 | 10 | |
| Reduction of peripheral neuropathy | Completed non-randomized clinical trial (stage II) NCT05884281 | 11 | |
| Improvement of fragile X syndrome compared to baclofen and metformin | Uncompleted randomized clinical trial (stage II) NCT05163808 | 12 | |
| Reduction of alcohol consumption in vitro | Completed non-randomized clinical trial (stage I).Unregistered in clinicaltrials.gov | 13 | |
| Digestive | Potential reduction of ulcerative colitis flares | Ongoing clinical trial (stage I) NCT05684484 | 81 |
| Evaluation of roflumilast vs pioglitazone in non-alcoholic steatohepatitis | Completed randomized clinical trial (stage II) NCT01703260 | 82 | |
| Endocrinological | Use of roflumilast in type II diabetes | Completed randomized clinical trial (stage II) NCT01140542 | 83 |
| Reduction of insulin and glucose levels in obese prediabetic patients | Completed non-randomized clinical trial (stage II) NCT01862029 | 41 | |
| Improvement of polycystic ovary syndrome compared to metformin | Completed non-randomized clinical trial (stage IV) NCT02037672 | 84 | |
| Reduction of diabetic neuropathy compared to alpha-lipoic acid | Completed non-randomized clinical trial (stage III) NCT05369793 | 85 | |
| Renal | Improvement of analytical parameters of diabetic nephropathy | Completed non-randomized clinical trial (stage III) NCT04755946 | 86 |
CB, chronic bronchitis; COPD, chronic obstructive pulmonary disease.
We conducted a narrative review of the scientific medical literature currently available. Searches were performed on Medline and Google Scholar in the months of May and June 2023 using the following keyterms: “roflumilast,” “dermatology,” “skin,” “off-label,” “safety,” “apremilast,” “phosphodiesterase,” “phosphodiesterase 4,” “psoriasis,” “atopy,” “atopic dermatitis,” “eczema,” “hand eczema,” “nummular eczema,” “ulcers,” “oral ulcers,” “aphthous stomatitis,” “oral aphthosis,” “lichen planus,” “seborrheic dermatitis,” “hidradenitis,” “hidradenitis suppurativa,” “vitiligo,” “alopecia areata,” “sarcoidosis,” “Behçet's,” and “morphea.” This search was conducted among articles both written in Spanish and English. These articles were screened based on their abstracts, and selected according to their relevance after reading the studies. Similarly, a search with the keyterm “roflumilast” was conducted on clinicaltrials.gov. Two authors (MMP, and DMC) performed the search and article selection.
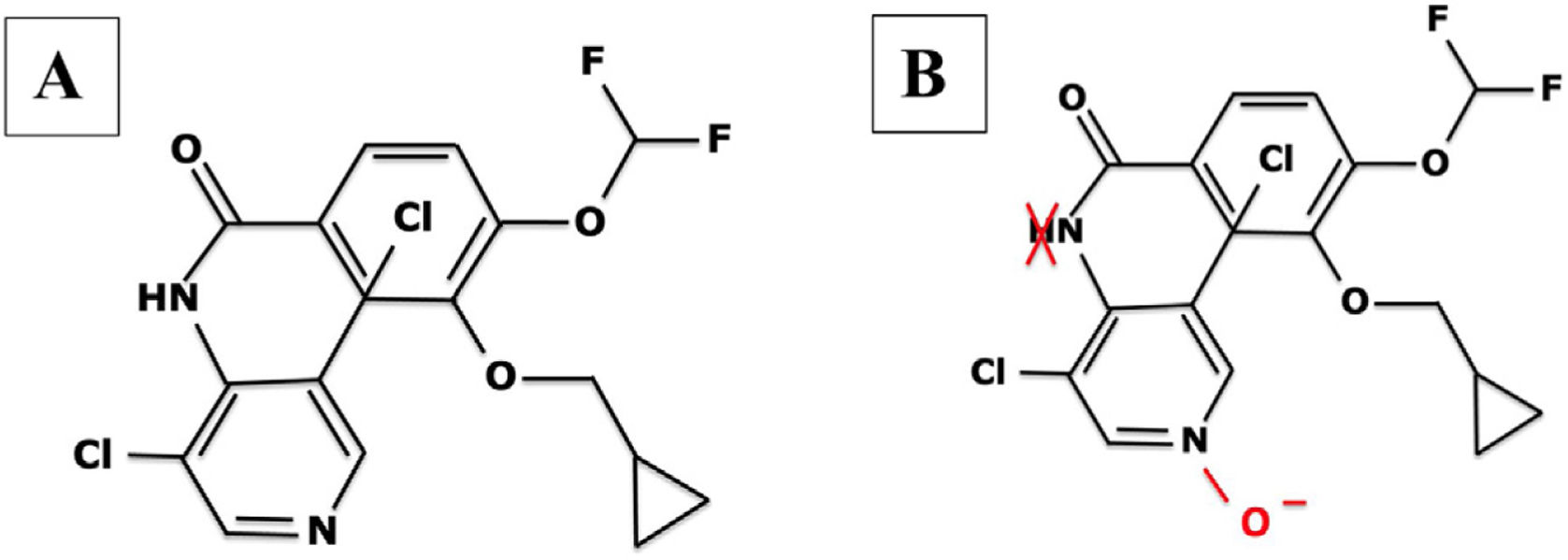
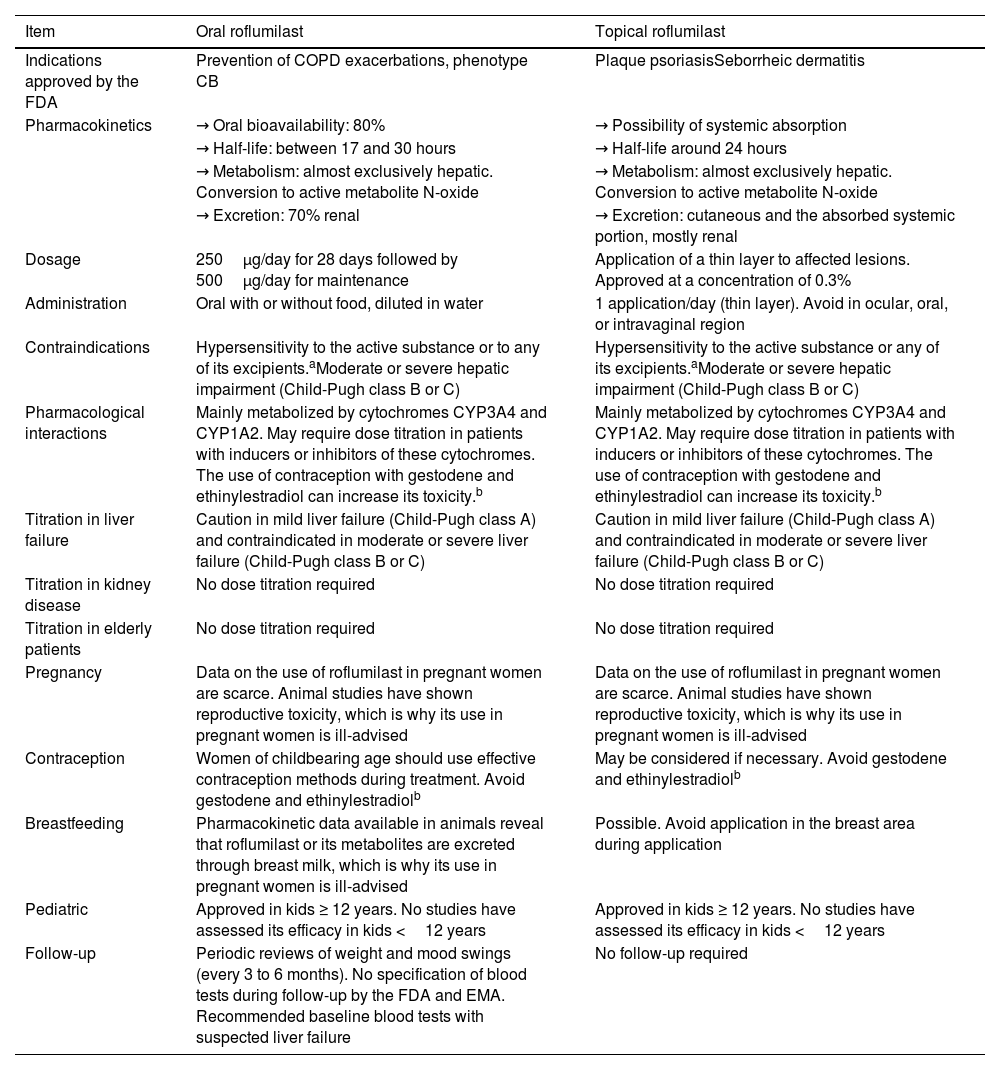
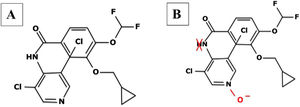
Roflumilast pharmacokinetics and mechanism of actionPharmacokinetics (table 2)When administered orally, roflumilast is completely absorbed by the GI tract, and its bioavailability stands at nearly 80%, reching its maximum plasma concentration within 1 hour after its administration.20 (table 2). Regarding its metabolism, roflumilast is mainly hepatically metabolized by cytochromes CYP1A2 and CYP3A4, which turn the initial compound into the active metabolite roflumilast N-oxide (figure 121), whih is >90% of roflumilast overall PDE4 inhibitory activity.22 The original and active metabolites of roflumilast mainly bind to plasma proteins (≥ 97%), and have a high volume of distribution, which is indicative of significant tissue penetration. No dose titration is required for geriatric age, or in cases of kidney disease. The clinical data available on roflumilast in patients with class A mild liver failure according to the Child-Pugh scale are not enough to recommend dose titration. Therefore, roflumilast should be used with caution in these individuals, and is contraindicated in moderate or severe liver failure.23 Roflumilast N-oxide is nearly 10 times more active than the original drug. The plasma half-life of roflumilast and its active metabolite is 17 to 30hours, respectively.24 Its excretion is mostly renal. While its pharmacokinetics is not affected by food intake, it could be affected by drugs that inhibit or induce CYP1A2 and CYP3A4, such as erythromycin, fluconazole, clarithromycin, or rifampicin.25,26 Contraception is advised in fertile women, avoiding contraceptives with gestodene and ethinylestradiol due to shared metabolism.25,26
Characteristics of roflumilast.8,38,41,80-86
| Item | Oral roflumilast | Topical roflumilast |
|---|---|---|
| Indications approved by the FDA | Prevention of COPD exacerbations, phenotype CB | Plaque psoriasisSeborrheic dermatitis |
| Pharmacokinetics | → Oral bioavailability: 80% | → Possibility of systemic absorption |
| → Half-life: between 17 and 30 hours | → Half-life around 24 hours | |
| → Metabolism: almost exclusively hepatic. Conversion to active metabolite N-oxide | → Metabolism: almost exclusively hepatic. Conversion to active metabolite N-oxide | |
| → Excretion: 70% renal | → Excretion: cutaneous and the absorbed systemic portion, mostly renal | |
| Dosage | 250μg/day for 28 days followed by 500μg/day for maintenance | Application of a thin layer to affected lesions. Approved at a concentration of 0.3% |
| Administration | Oral with or without food, diluted in water | 1 application/day (thin layer). Avoid in ocular, oral, or intravaginal region |
| Contraindications | Hypersensitivity to the active substance or to any of its excipients.aModerate or severe hepatic impairment (Child-Pugh class B or C) | Hypersensitivity to the active substance or any of its excipients.aModerate or severe hepatic impairment (Child-Pugh class B or C) |
| Pharmacological interactions | Mainly metabolized by cytochromes CYP3A4 and CYP1A2. May require dose titration in patients with inducers or inhibitors of these cytochromes. The use of contraception with gestodene and ethinylestradiol can increase its toxicity.b | Mainly metabolized by cytochromes CYP3A4 and CYP1A2. May require dose titration in patients with inducers or inhibitors of these cytochromes. The use of contraception with gestodene and ethinylestradiol can increase its toxicity.b |
| Titration in liver failure | Caution in mild liver failure (Child-Pugh class A) and contraindicated in moderate or severe liver failure (Child-Pugh class B or C) | Caution in mild liver failure (Child-Pugh class A) and contraindicated in moderate or severe liver failure (Child-Pugh class B or C) |
| Titration in kidney disease | No dose titration required | No dose titration required |
| Titration in elderly patients | No dose titration required | No dose titration required |
| Pregnancy | Data on the use of roflumilast in pregnant women are scarce. Animal studies have shown reproductive toxicity, which is why its use in pregnant women is ill-advised | Data on the use of roflumilast in pregnant women are scarce. Animal studies have shown reproductive toxicity, which is why its use in pregnant women is ill-advised |
| Contraception | Women of childbearing age should use effective contraception methods during treatment. Avoid gestodene and ethinylestradiolb | May be considered if necessary. Avoid gestodene and ethinylestradiolb |
| Breastfeeding | Pharmacokinetic data available in animals reveal that roflumilast or its metabolites are excreted through breast milk, which is why its use in pregnant women is ill-advised | Possible. Avoid application in the breast area during application |
| Pediatric | Approved in kids ≥ 12 years. No studies have assessed its efficacy in kids <12 years | Approved in kids ≥ 12 years. No studies have assessed its efficacy in kids <12 years |
| Follow-up | Periodic reviews of weight and mood swings (every 3 to 6 months). No specification of blood tests during follow-up by the FDA and EMA. Recommended baseline blood tests with suspected liver failure | No follow-up required |
CB, chronic bronchitis; COPD, chronic obstructive pulmonary disease.
The pharmacokinetics of 0.3% topical roflumilast cream is similar to that of oral roflumilast, taking into consideration the possibility of systemic absorption. Topically, its bioavailability is nearly 1.5%. After applying 3-to-6.5 grams/day for 15 days, it showed a mean exposure of 72.7 ± 53.1 and 628 ± 648hours ng/mL.7,27
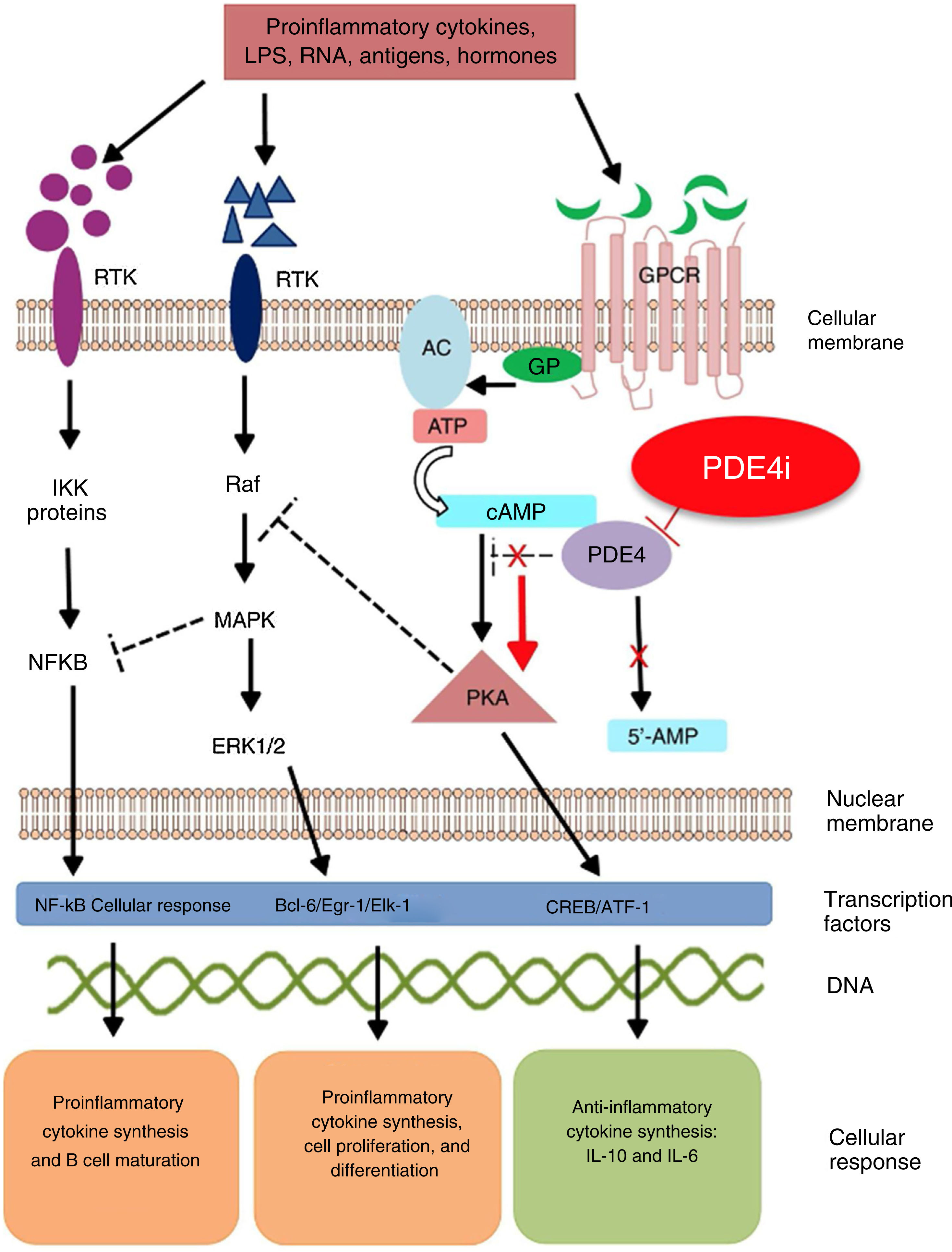
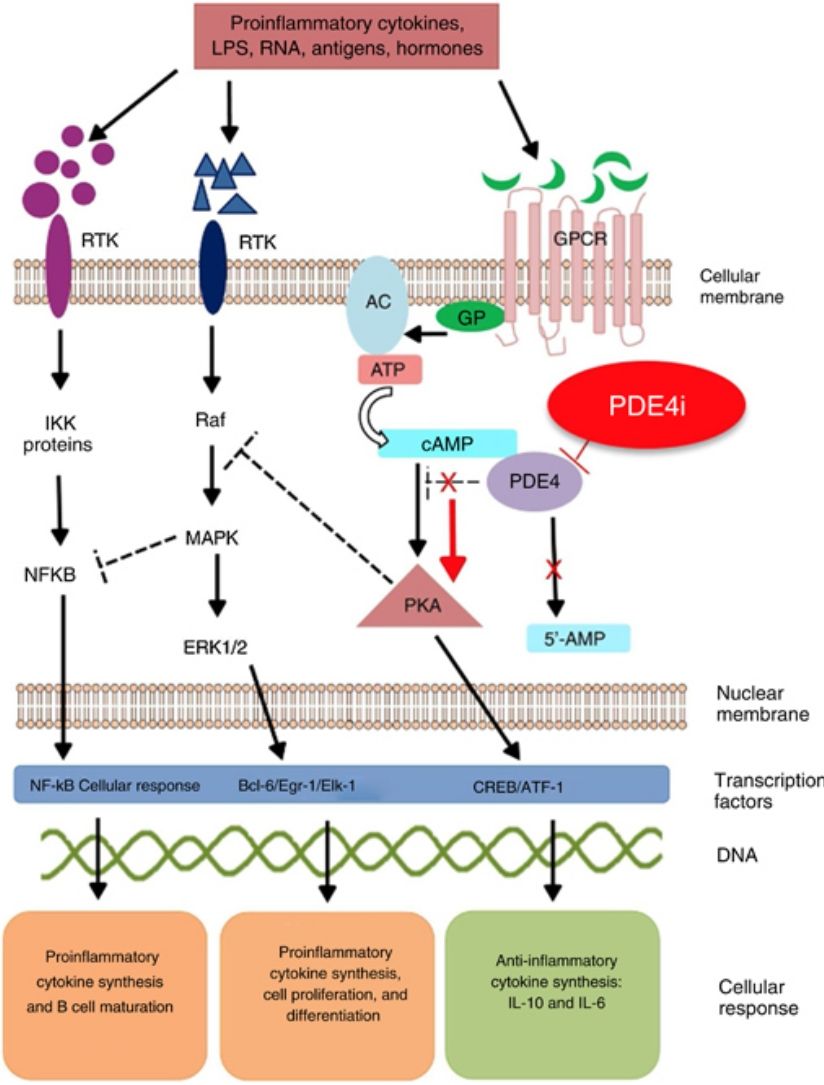
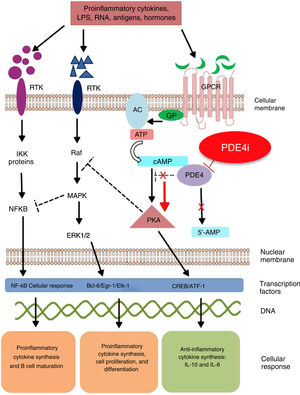
Mechanism of action and pharmacodynamicsThe exact mechanism of action of roflumilast is still to be elucidated (figure 2). Roflumilast and its active metabolite, roflumilast N-oxide, are PDE4 inhibitors (PDE4i), which happen to be the main enzymes involved in the metabolism of cyclic adenosine monophosphate (cAMP) found in lung tissue, skin, heart, kidneys, GI tract, and nervous system.28 At cellular level, PDE4 turns cAMP into adenosine monophosphate (AMP), thereby terminating the cellular messaging started by cAMP.29 Roflumilast blocks the effect of PDE4, thus leading to the accumulation of cAMP in target cells, and increasing the signaling mediated by this molecule. This results in the inhibition of chemotaxis, reduction of inflammatory infiltration, decreased release of inflammatory and cytotoxic mediators, and an overal reduction of inflammation.30 In dermatology, PDE4 is present in epidermal keratinocytes, neutrophils, Langerhans cells, and T lymphocytes.31 Additionally, high levels of PDE4 have been found in peripheral blood mononuclear cells of patients with psoriasis, along with changes to ATP signaling.32 The effectiveness of roflumilast is based on the inhibition of multiple inflammatory pathways, acting at epidermal level in keratinocytes and Langerhans cells, and dermal level in neutrophils, T lymphocytes, and macrophages.30,33 In atopic dermatitis, the hyperactivation of PDE4 induces an inflammatory response with polarization towards the Th2 pathway.31,33 Specifically, cAMP has immunosuppressive and anti-inflammatory properties, which are mostly mediated by the nuclear factor-κB, which is key for the activity of multiple cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukins (IL) 1, 2, 8, 12, 18, 23, 27, or 36.34,35 The multiple PDE4-mediate signaling pathways suggest that its blockade may be useful to trest various dermatoses, such as hidradenitis suppurativa, recurrent aphthous stomatitis, or lichen planus.3
Mechanism of action of roflumilast. Phosphodiesterase inhibitors lead to an accumulation of intracellular cAMP by interfering with its degradation. The increased intracellular concentration of cAMP results in the inhibition of chemotaxis, reduced inflammatory cell infiltration, and decreased release of inflammatory and cytotoxic mediators, thereby reducing inflammation.
5’-AMP, 5’-adenylic acid; AC, adenylate cyclase; ATF, activating transcription factor 1; ATP, adenosine triphosphate; Bcl-6, B-cell lymphoma protein 6; c-AMP, cyclic adenosine monophosphate; CREB, cAMP responsive element; Egr-1, early growth response protein 1; Elk-1, E-26-like protein 1; ERK, extracellular signal-regulated kinase; GP, G protein; GPCR, G protein-coupled receptors; IKKbeta, inhibitor of nuclear factor kappa-B kinase subunit beta; IPDE-4, inhibitor of phosphodiesterase type 4; MAPK, mitogen-activated protein kinases; NFKB, nuclear factor KB; PDE4, phosphodiesterase type 4; PDE4i, phosphodiesterase type 4 inhibitor; PKA, protein kinase A; Raf, rapidly accelerated fibrosarcoma protein kinases; RTK, receptor tyrosine kinases.34
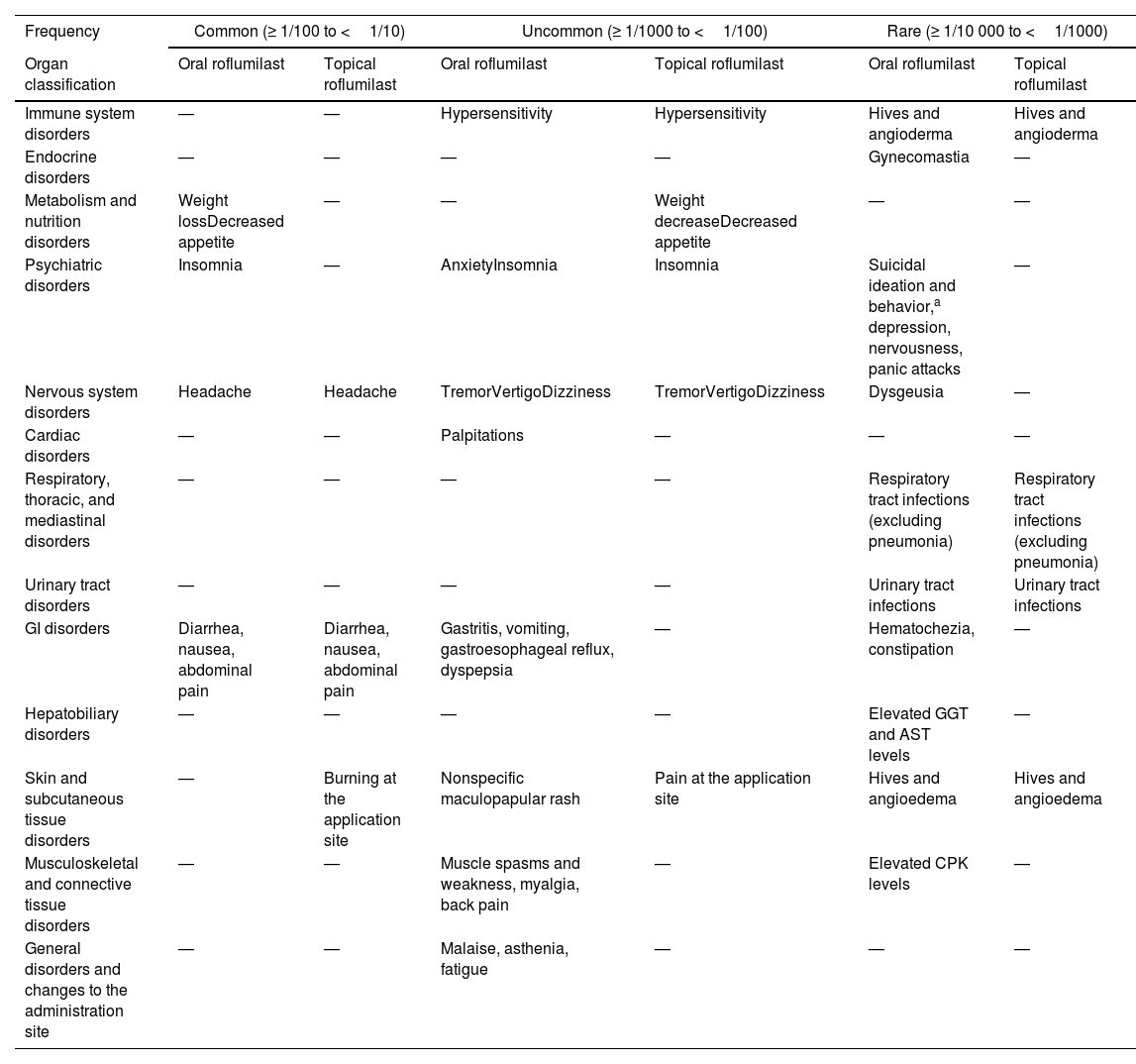
Overall, roflumilast is well-tolerated and has an excellent safety profile24 (table 3). In clinical trials (CT) on roflumilast to treat COPD and chronic bronchitis, the most common adverse event (AE) reported were diarrhea (8% to 9%), weight loss (6% to 12%), and nausea (5%). The incidence of nasopharyngitis (5% to 8%) and upper respiratory tract infections (4%) has been reported. However, these data are similar to those from the placebo group. AEs were mainly reported within the first weeks of treatment and were mostly self-limiting.28 A meta-analysis reported a higher rate of AEs in the roflumilast 500μg/day group vs placebo.36 A total of 15% discontinued treatment due to AEs (compared to 9% from the placebo group). The most common reasons for drug discontinuation were diarrhea and nausea.28 These initial analyses also reported higher rates of acute pancreatitis, psychiatric symptoms, and even prostate, lung, and colorectal cancer.37 However, these initial findings could not be confirmed later, and roflumilast has even been proposed as an adjuvant therapy to treat various neoplasms, such as lung cancer or diffuse large B-cell lymphoma.38,39
Adverse events associated with the use of roflumilast.
| Frequency | Common (≥ 1/100 to <1/10) | Uncommon (≥ 1/1000 to <1/100) | Rare (≥ 1/10 000 to <1/1000) | |||
|---|---|---|---|---|---|---|
| Organ classification | Oral roflumilast | Topical roflumilast | Oral roflumilast | Topical roflumilast | Oral roflumilast | Topical roflumilast |
| Immune system disorders | — | — | Hypersensitivity | Hypersensitivity | Hives and angioderma | Hives and angioderma |
| Endocrine disorders | — | — | — | — | Gynecomastia | — |
| Metabolism and nutrition disorders | Weight lossDecreased appetite | — | — | Weight decreaseDecreased appetite | — | — |
| Psychiatric disorders | Insomnia | — | AnxietyInsomnia | Insomnia | Suicidal ideation and behavior,a depression, nervousness, panic attacks | — |
| Nervous system disorders | Headache | Headache | TremorVertigoDizziness | TremorVertigoDizziness | Dysgeusia | — |
| Cardiac disorders | — | — | Palpitations | — | — | — |
| Respiratory, thoracic, and mediastinal disorders | — | — | — | — | Respiratory tract infections (excluding pneumonia) | Respiratory tract infections (excluding pneumonia) |
| Urinary tract disorders | — | — | — | — | Urinary tract infections | Urinary tract infections |
| GI disorders | Diarrhea, nausea, abdominal pain | Diarrhea, nausea, abdominal pain | Gastritis, vomiting, gastroesophageal reflux, dyspepsia | — | Hematochezia, constipation | — |
| Hepatobiliary disorders | — | — | — | — | Elevated GGT and AST levels | — |
| Skin and subcutaneous tissue disorders | — | Burning at the application site | Nonspecific maculopapular rash | Pain at the application site | Hives and angioedema | Hives and angioedema |
| Musculoskeletal and connective tissue disorders | — | — | Muscle spasms and weakness, myalgia, back pain | — | Elevated CPK levels | — |
| General disorders and changes to the administration site | — | — | Malaise, asthenia, fatigue | — | — | — |
—: Not described or anecdotal reports; AST, aspartate aminotransferase; CPK, creatine phosphokinase; GGT, gamma-glutamyltransferase.
In clinical trials and post-marketing surveillance, rare cases of suicidal ideation and behavior, including suicide, have been reported.37,43
Regarding weight loss, its incidence was nearly double (67.4% vs 37.7%) in the roflumilast group, and weight loss turned out to be significant (> 10% compared to baseline in 7.1% of the treatment group vs 1.9% of the control group).37 This significant weight loss was associated with an improved glycemic metabolic profile and constitutes a potential therapeutic approach for the management of obesity, insulin resistance, and metabolic syndrome.40,41 The psychiatric symptoms included in the initial studies were anxiety, depression, and insomnia.28 However, subsequent studies have confirmed that this risk is minimal, and in fact, roflumilast could be a promising drug to treat cognitive impairment, Alzheimer's disease, or schizophrenia.8,10,42 Finally, the increased incidence of atrial fibrillation in patients on oral roflumilast has been a matter of discussion due to its higher incidence rate in the oral roflumilast group (n=24) compared to the placebo group (n=9) in pre-commercialization trials. However, this has not been confirmed in the routine clinical practice. Additionally, the results of 24-hour Holter EKG monitoring in 55 patients showed no inter-gropu differences regarding heart rate or the occurrence of arrhythmias.43
Long-term safety data with PDE4i, specifically roflumilast, are equally favorable, with no new AEs or cumulative AEs being reported.44,45 AEs have not been described either in diseases in which this drug is commonly used, such as COPD.46 Currently, there is an ongoing clinical trial to assess the long-term safety profile of oral roflumilast to treat COPD.47
The safety profile of topical roflumilast cream is also favorable, with exceptionally rare serious AE being reported due to its minimal absorption (bioavailability of 1% to 2%). The most common AEs reported include diarrhea (3% to 4%) and headache (2% to 4%), followed by insomnia, nausea, itching, or discomfort at the application site.7,27
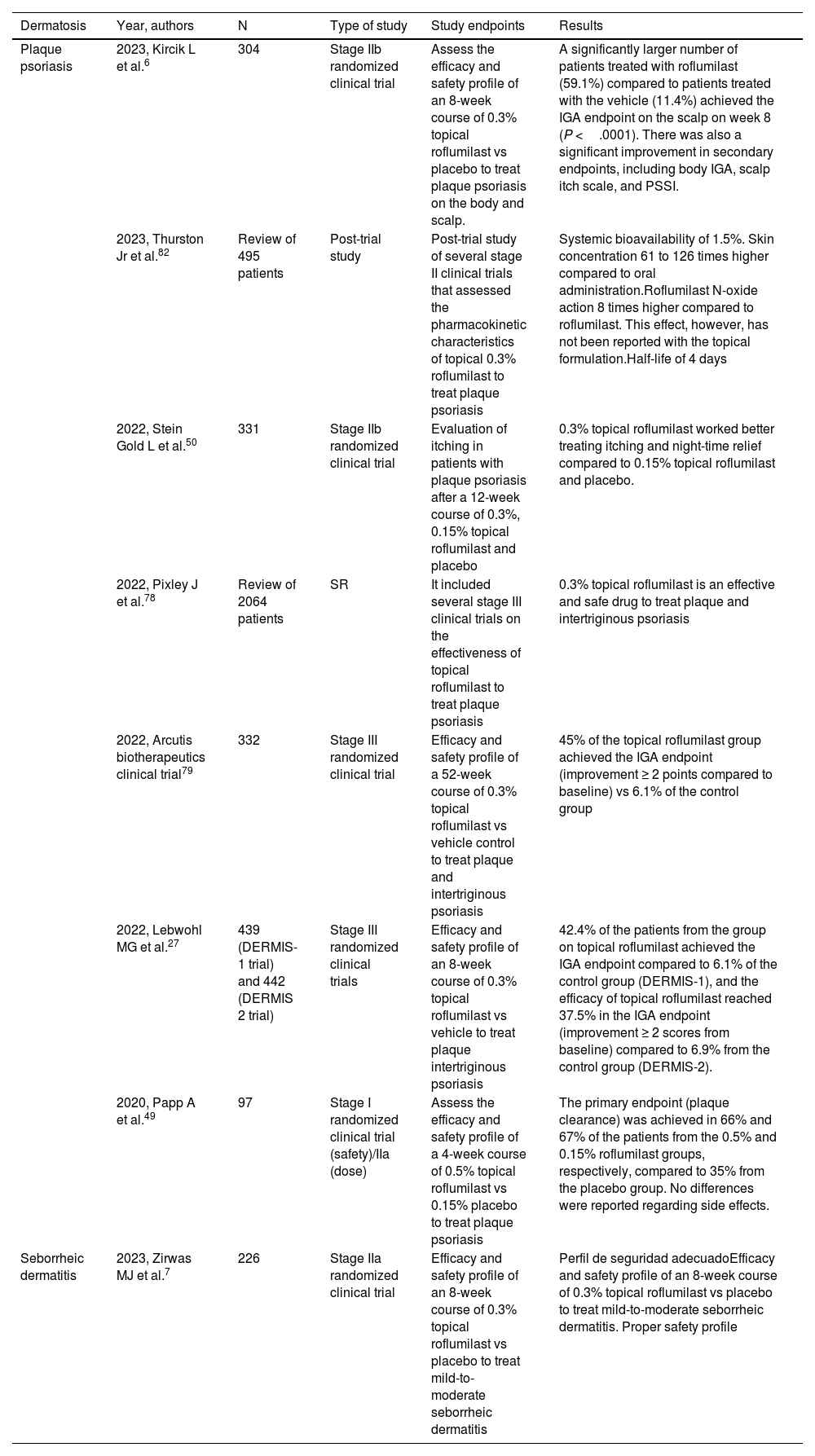
Approved indications in dermatologyThe use of oral roflumilast has been approved by the FDA (unlike the EMA) for the topical treatment of plaque psoriasis and mild-to-moderate seborrheic dermatitis.6,7,27 The first report on roflumilast in the management of plaque psoriasis dates back to 2017 when Michels et al.48 described a case of psoriasis and concomitant COPD in which skin lesions improved while lung disease was being treated with oral roflumilast. In 2020, Papp et al.49 reported the first stage I/IIa clinical trial (CT). This trial confirmed that a response was achieved when plaque psoriasis was treated with 0.5% and 0.15% topical roflumilast cream, which was superior compared to the administration of placebo. The DERMIS-1 and DERMIS-2 trials27 led to the FDA approval of 0.3% topical roflumilast to treat plaque psoriasis, with 42.4% of cases achieving the Investigator's Global Assessment Scale (IGA) endpoint of complete clearance or improved IGA ≥ 2 points compared to baseline vs 6.1% of the patients from the control group (DERMIS-1), and 37.5% achieving the IGA endpoint compared to 6.9% from the control group (DERMIS-2). The safety profile was favorable in both studies, being GI discomfort the most common AE of all.77 Recently, 2 stage IIb50 and stage III6 trials have been published confirming the efficacy profile of 0.3% topical roflumilast to treat pruritus, nocturnal rest, and scalp involvement, respectively (table 4).
Studies on topical roflumilast to treat plaque psoriasis and seborrheic dermatitis.6,7,27,49,50,78,79,82
| Dermatosis | Year, authors | N | Type of study | Study endpoints | Results |
|---|---|---|---|---|---|
| Plaque psoriasis | 2023, Kircik L et al.6 | 304 | Stage IIb randomized clinical trial | Assess the efficacy and safety profile of an 8-week course of 0.3% topical roflumilast vs placebo to treat plaque psoriasis on the body and scalp. | A significantly larger number of patients treated with roflumilast (59.1%) compared to patients treated with the vehicle (11.4%) achieved the IGA endpoint on the scalp on week 8 (P <.0001). There was also a significant improvement in secondary endpoints, including body IGA, scalp itch scale, and PSSI. |
| 2023, Thurston Jr et al.82 | Review of 495 patients | Post-trial study | Post-trial study of several stage II clinical trials that assessed the pharmacokinetic characteristics of topical 0.3% roflumilast to treat plaque psoriasis | Systemic bioavailability of 1.5%. Skin concentration 61 to 126 times higher compared to oral administration.Roflumilast N-oxide action 8 times higher compared to roflumilast. This effect, however, has not been reported with the topical formulation.Half-life of 4 days | |
| 2022, Stein Gold L et al.50 | 331 | Stage IIb randomized clinical trial | Evaluation of itching in patients with plaque psoriasis after a 12-week course of 0.3%, 0.15% topical roflumilast and placebo | 0.3% topical roflumilast worked better treating itching and night-time relief compared to 0.15% topical roflumilast and placebo. | |
| 2022, Pixley J et al.78 | Review of 2064 patients | SR | It included several stage III clinical trials on the effectiveness of topical roflumilast to treat plaque psoriasis | 0.3% topical roflumilast is an effective and safe drug to treat plaque and intertriginous psoriasis | |
| 2022, Arcutis biotherapeutics clinical trial79 | 332 | Stage III randomized clinical trial | Efficacy and safety profile of a 52-week course of 0.3% topical roflumilast vs vehicle control to treat plaque and intertriginous psoriasis | 45% of the topical roflumilast group achieved the IGA endpoint (improvement ≥ 2 points compared to baseline) vs 6.1% of the control group | |
| 2022, Lebwohl MG et al.27 | 439 (DERMIS-1 trial) and 442 (DERMIS 2 trial) | Stage III randomized clinical trials | Efficacy and safety profile of an 8-week course of 0.3% topical roflumilast vs vehicle to treat plaque intertriginous psoriasis | 42.4% of the patients from the group on topical roflumilast achieved the IGA endpoint compared to 6.1% of the control group (DERMIS-1), and the efficacy of topical roflumilast reached 37.5% in the IGA endpoint (improvement ≥ 2 scores from baseline) compared to 6.9% from the control group (DERMIS-2). | |
| 2020, Papp A et al.49 | 97 | Stage I randomized clinical trial (safety)/IIa (dose) | Assess the efficacy and safety profile of a 4-week course of 0.5% topical roflumilast vs 0.15% placebo to treat plaque psoriasis | The primary endpoint (plaque clearance) was achieved in 66% and 67% of the patients from the 0.5% and 0.15% roflumilast groups, respectively, compared to 35% from the placebo group. No differences were reported regarding side effects. | |
| Seborrheic dermatitis | 2023, Zirwas MJ et al.7 | 226 | Stage IIa randomized clinical trial | Efficacy and safety profile of an 8-week course of 0.3% topical roflumilast vs placebo to treat mild-to-moderate seborrheic dermatitis | Perfil de seguridad adecuadoEfficacy and safety profile of an 8-week course of 0.3% topical roflumilast vs placebo to treat mild-to-moderate seborrheic dermatitis. Proper safety profile |
COPD, chronic obstructive pulmonary disease; IGA, Investigator's Global Assessment Scale; PSSI, Psoriasis Scalp Severity Index; SR, systematic review.
The FDA approval of topical roflumilast to treat seborrheic dermatitis was granted back in April 2023, following the publication of the results from a multicenter, placebo-controlled stage IIa CT on the efficacy and safety profile of 0.3% roflumilast in 226 patients with a >3-month history of seborrheic dermatitis and IGA scores ≥ 3 (≥ moderate) with involvement of <20% of the body surface area, including the scalp, face, trunk, and intertriginous areas.7 The trial achieved the target IGA (≥ 2-point clearance from baseline) in 73.8% of the patients on roflumilast, compared to 40.9% of the patients from the control group (P <.001). The difference was significant from the 2nd week of treatment. No higher rate of AEs was ever reported in the roflumilast group compared to the control one.
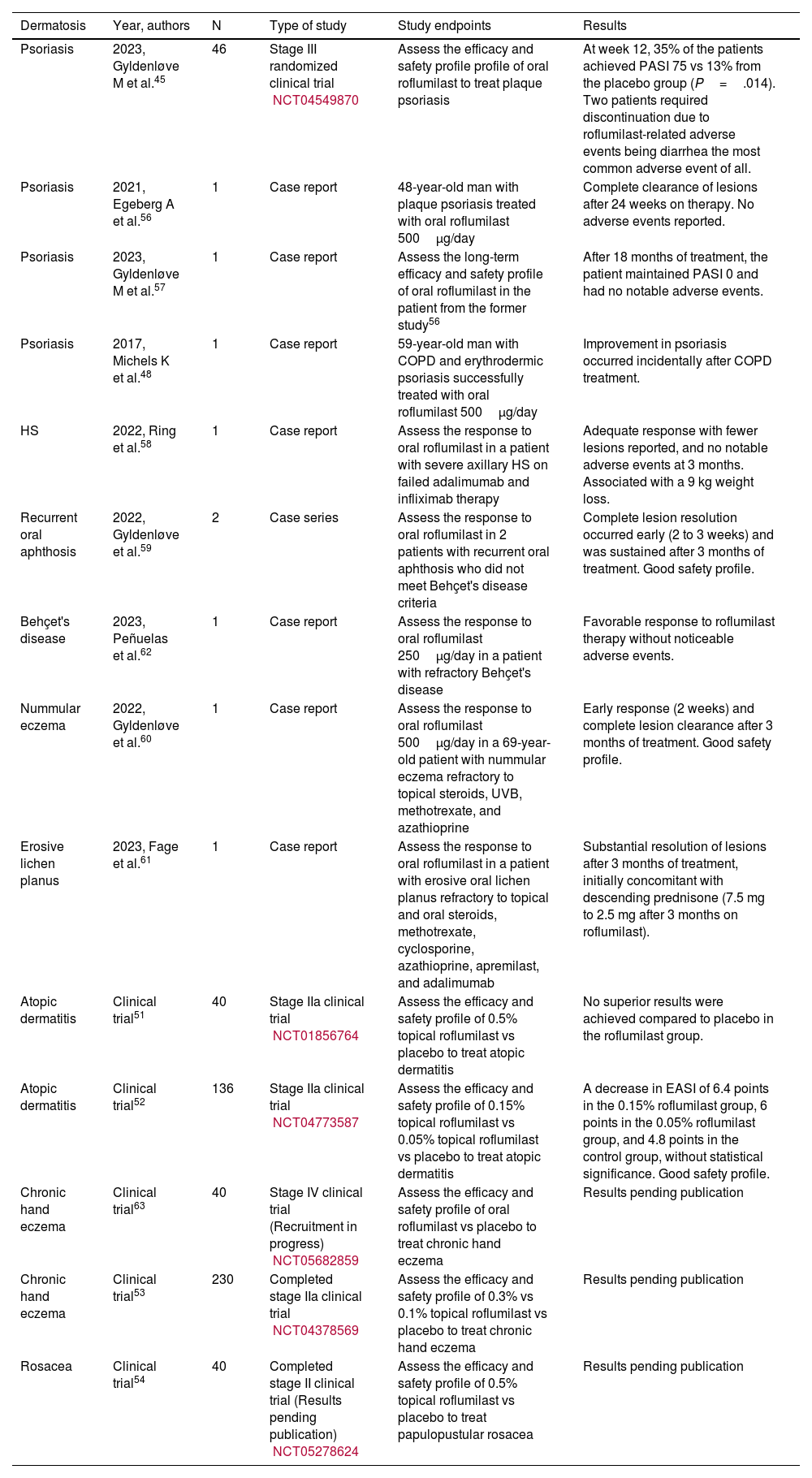
Off-label use of roflumilast in dermatologyThe medical literature available includes various trials (most of them small series of cases or initial CT) on the off-label use of roflumilast in dermatology (table 5). Specifically, we found 4 clinical trials on the use of topical roflumilast. Regarding atopic dermatitis, one stage IIa CT (n=40) failed to achieve its proposed endpoints with 0.5% topical roflumilast being compared to placebo at 16 weeks. The primary endpoint of this trial was to determine the number of patients who achieved a reduction of, at least, 75% in the Hand Eczema Severity Index (HECSI75) after treatment with roflumilast topical cream.51 Another randomized stage IIa clinical trial (n=136) showed an improved Eczema Area and Severity Index (EASI) score at 12 weeks with 0.15% topical roflumilast (superior to that obtained with the 0.05% concentration and placebo). However, these results did not reach statistical significance.52 Two currently ongoing clinical trials on the effects of topical roflumilast to treat chronic hand eczema,53 and papulopustular rosacea.54 The CT on chronic hand eczema (n=230) is stage IIa trial on the efficacy and safety profile of topical roflumilast cream at 0.3% vs 0.1% vs placebo. The trial will be assessed at week 12. The CT on papulopustular rosacea (n=40) has already been completed and is currently pending publication. It evaluates the efficacy profile of 0.5% topical roflumilast vs placebo. Recently, a preclinical trial found higher levels of PDE4 in the skin of patients with vitiligo. After the application of topical roflumilast, the PDE4 levels dropped, and skin lesions improved partially.55
Clinical trials on the off-label use of roflumilast in dermatology.45,48,51-54,56-63
| Dermatosis | Year, authors | N | Type of study | Study endpoints | Results |
|---|---|---|---|---|---|
| Psoriasis | 2023, Gyldenløve M et al.45 | 46 | Stage III randomized clinical trial NCT04549870 | Assess the efficacy and safety profile profile of oral roflumilast to treat plaque psoriasis | At week 12, 35% of the patients achieved PASI 75 vs 13% from the placebo group (P=.014). Two patients required discontinuation due to roflumilast-related adverse events being diarrhea the most common adverse event of all. |
| Psoriasis | 2021, Egeberg A et al.56 | 1 | Case report | 48-year-old man with plaque psoriasis treated with oral roflumilast 500μg/day | Complete clearance of lesions after 24 weeks on therapy. No adverse events reported. |
| Psoriasis | 2023, Gyldenløve M et al.57 | 1 | Case report | Assess the long-term efficacy and safety profile of oral roflumilast in the patient from the former study56 | After 18 months of treatment, the patient maintained PASI 0 and had no notable adverse events. |
| Psoriasis | 2017, Michels K et al.48 | 1 | Case report | 59-year-old man with COPD and erythrodermic psoriasis successfully treated with oral roflumilast 500μg/day | Improvement in psoriasis occurred incidentally after COPD treatment. |
| HS | 2022, Ring et al.58 | 1 | Case report | Assess the response to oral roflumilast in a patient with severe axillary HS on failed adalimumab and infliximab therapy | Adequate response with fewer lesions reported, and no notable adverse events at 3 months. Associated with a 9 kg weight loss. |
| Recurrent oral aphthosis | 2022, Gyldenløve et al.59 | 2 | Case series | Assess the response to oral roflumilast in 2 patients with recurrent oral aphthosis who did not meet Behçet's disease criteria | Complete lesion resolution occurred early (2 to 3 weeks) and was sustained after 3 months of treatment. Good safety profile. |
| Behçet's disease | 2023, Peñuelas et al.62 | 1 | Case report | Assess the response to oral roflumilast 250μg/day in a patient with refractory Behçet's disease | Favorable response to roflumilast therapy without noticeable adverse events. |
| Nummular eczema | 2022, Gyldenløve et al.60 | 1 | Case report | Assess the response to oral roflumilast 500μg/day in a 69-year-old patient with nummular eczema refractory to topical steroids, UVB, methotrexate, and azathioprine | Early response (2 weeks) and complete lesion clearance after 3 months of treatment. Good safety profile. |
| Erosive lichen planus | 2023, Fage et al.61 | 1 | Case report | Assess the response to oral roflumilast in a patient with erosive oral lichen planus refractory to topical and oral steroids, methotrexate, cyclosporine, azathioprine, apremilast, and adalimumab | Substantial resolution of lesions after 3 months of treatment, initially concomitant with descending prednisone (7.5 mg to 2.5 mg after 3 months on roflumilast). |
| Atopic dermatitis | Clinical trial51 | 40 | Stage IIa clinical trial NCT01856764 | Assess the efficacy and safety profile of 0.5% topical roflumilast vs placebo to treat atopic dermatitis | No superior results were achieved compared to placebo in the roflumilast group. |
| Atopic dermatitis | Clinical trial52 | 136 | Stage IIa clinical trial NCT04773587 | Assess the efficacy and safety profile of 0.15% topical roflumilast vs 0.05% topical roflumilast vs placebo to treat atopic dermatitis | A decrease in EASI of 6.4 points in the 0.15% roflumilast group, 6 points in the 0.05% roflumilast group, and 4.8 points in the control group, without statistical significance. Good safety profile. |
| Chronic hand eczema | Clinical trial63 | 40 | Stage IV clinical trial (Recruitment in progress) NCT05682859 | Assess the efficacy and safety profile of oral roflumilast vs placebo to treat chronic hand eczema | Results pending publication |
| Chronic hand eczema | Clinical trial53 | 230 | Completed stage IIa clinical trial NCT04378569 | Assess the efficacy and safety profile of 0.3% vs 0.1% topical roflumilast vs placebo to treat chronic hand eczema | Results pending publication |
| Rosacea | Clinical trial54 | 40 | Completed stage II clinical trial (Results pending publication) NCT05278624 | Assess the efficacy and safety profile of 0.5% topical roflumilast vs placebo to treat papulopustular rosacea | Results pending publication |
HS, hidradenitis suppurativa; UVB, ultraviolet-B Radiation therapy.
Regarding the off-label use of oral roflumilast, we found 4 trials on the off-labe use of roflumilast to treat psoriasis (table 5). Gyldenløve et al. have recently reported the efficacy of 500μg/day of oral roflumilast to treat plaque psoriasis in a stage III CT (n=46) vs placebo, with 35% of the intervention group achieving PASI 75 vs 13% of the placebo group (P=.014) at week 12. Mostly mild AEs were reported.45 Recently, the same group published the results of oral roflumilast in the first patient with refractory plaque psoriasis in whom this drug was ever prescribed, a 48-year-old man.56 They later reported on the long-term disease progression (18 months) of this individual.57 Recently, its utility has also been described in another clinical case: a 59-year-old man who was simultaneously being treated for COPD and psoriasis.48 In both cases, roflumilast was used at a dose of 500μg/day, with no significant adverse events being reported. Aside from psoriasis, the scientific medical literature currently available includes 5 articles on oral roflumilast in dermatological disease (table 5): 1 case series and 4 isolated clinical cases, most of them reported by the same health care center in Denmark. Ring et al. described 1 case of refractory hidradenitis suppurativa with axillary involvement, failed adalimumab and infliximab, and good clinical response to roflumilast at 12 weeks, with associated weight loss.58 Gyldenløve et al. reported 2 cases of recurrent oral aphthosis treated with oral roflumilast 500μg/day, showing proper and rapid responses within 2 to 3 weeks and maintained responses after 3 months of treatment.59 Similarly, they also described 1 case of nummular eczema with failed multiple treatments that, somehow, achieved an early and sustained response to oral roflumilast, without visible adverse events being reported.60 There was also 1 case of erosive oral lichen planus refractory to first-line therapies and on systemic corticosteroids. Oral roflumilast allowed for down-titration of prednisone to 2.5mg/day at 3 months.61 A Spanish group recently reported 1 case of refractory Behçet's disease successfully treated with roflumilast 250μg/day, without significant adverse events being reported.62 Finally, a stage IV clinical trial (n=40) currently in the recruitment stage is evaluating oral roflumilast at a dose of 500μg/day vs placebo to treat chronic hand eczema.63
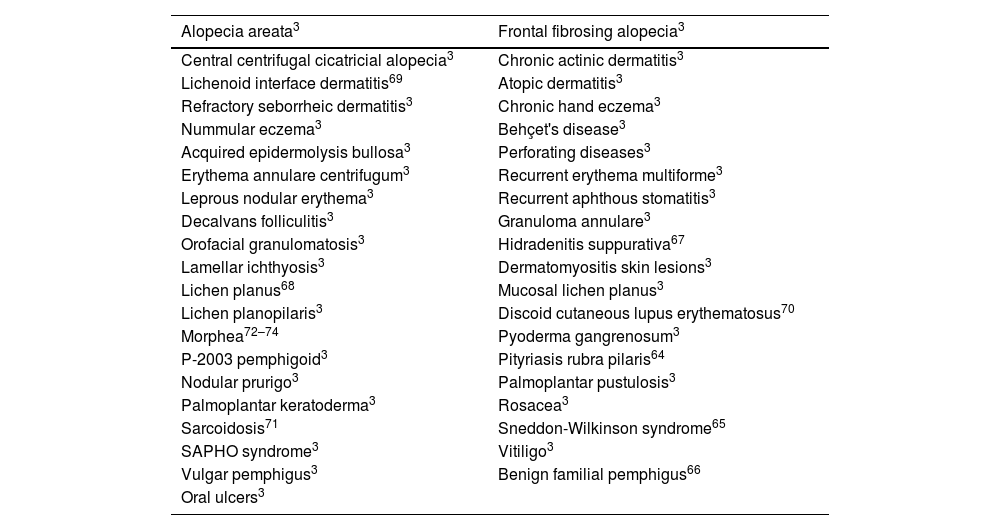
Dermatoses that could potentially benefit from roflumilastGiven its mechanism of action, which is similar to that of apremilast and the possibility of elevated PDE4 levels, multiple dermatoses in which this drug has been used off-label could potentially benefit from oral or topical roflumilast (table 6). Among these we find primarily autoimmune dermatoses like vitiligo or alopecia areata,3 erythematosquamous diseases similar to psoriasis such as pityriasis rubra pilaris,64 or Sneddon-Wilkinson syndrome,65 blistering diseases such as vulgar pemphigus or acquired epidermolysis bullosa,3 genodermatoses such as familial benign pemphigus (Hailey-Hailey disease),66 or ichthyosis.3 Other diseases where apremilast could also be useful include hidradenitis suppurativa,67 cutaneous lichen planus,68 or other lichenoid and interface dermatitis,69 cutaneous lupus erythematosus70, or cutaneous sarcoidosis.71 Its potential application in morphea, a relatively common disease where PDE4i have shown to reduce dermal fibrosis is of special interest. In fact, good results have been reported with apremilast to treat this entity.72–74
Off-label use of apremilast and potential uses of roflumilast in dermatology.
| Alopecia areata3 | Frontal fibrosing alopecia3 |
|---|---|
| Central centrifugal cicatricial alopecia3 | Chronic actinic dermatitis3 |
| Lichenoid interface dermatitis69 | Atopic dermatitis3 |
| Refractory seborrheic dermatitis3 | Chronic hand eczema3 |
| Nummular eczema3 | Behçet's disease3 |
| Acquired epidermolysis bullosa3 | Perforating diseases3 |
| Erythema annulare centrifugum3 | Recurrent erythema multiforme3 |
| Leprous nodular erythema3 | Recurrent aphthous stomatitis3 |
| Decalvans folliculitis3 | Granuloma annulare3 |
| Orofacial granulomatosis3 | Hidradenitis suppurativa67 |
| Lamellar ichthyosis3 | Dermatomyositis skin lesions3 |
| Lichen planus68 | Mucosal lichen planus3 |
| Lichen planopilaris3 | Discoid cutaneous lupus erythematosus70 |
| Morphea72–74 | Pyoderma gangrenosum3 |
| P-2003 pemphigoid3 | Pityriasis rubra pilaris64 |
| Nodular prurigo3 | Palmoplantar pustulosis3 |
| Palmoplantar keratoderma3 | Rosacea3 |
| Sarcoidosis71 | Sneddon-Wilkinson syndrome65 |
| SAPHO syndrome3 | Vitiligo3 |
| Vulgar pemphigus3 | Benign familial pemphigus66 |
| Oral ulcers3 |
SAPHO, synovitis acne pustulosis hyperostosis osteitis.
The management of moderate-to-severe inflammatory dermatoses often requires the use of immunomodulators or immunosuppressants, which are often used off-label in dermatology. Despite the addition of small molecules and biologic agents to our therapeutic arsenal, there is a need for new cost-effective immunomodulatory therapies with a good safety profile.75–77 One significant advantage of roflumilast is that it is not expensive (nearly €30/month in Spain for the oral formulation of 500μg/day, which is similar to the price reported in other countries).59 In fact, its price is lower than that of classic immunosuppressants such as oral cyclosporine or subcutaneous methotrexate. Another advantage is its excellent safety profile, with few contraindications and mostly GI AEs, which is very similar to what has already been reported with apremilast. Regarding serious AEs, a still-to-be elucidated relationship has been suggested on the use of roflumilast and atrial fibrillation. The association between roflumilast and suicide is also controversial and has not been found in subsequent studies.37,43 This drug has been on the market for over 10 years. During this time no new AEs or deleterious cumulative effects have ever been reported from its long-term use.44,45 However, GI AEs can be very bothersome and lead to its discontinuation in a significant number of patients. Therefore, proper titration is essential, with gradual up-titration. Overall, it is recommended to start at a dose of 250μg/day with or without food and always at the same time of day. With proper responses and in the absence of significant AEs, up-titrating to the optimal dose of 500μg/day is advised. Its use has been tested for up to 1 year, although it may be continued for longer periods of time at the physician's discretion.23
Convenient dosing and the possibility of dual application, both topically and orally are among the advantages of roflumilast.78 Additionally, it may lead to weight loss associated with an improved metabolic profile and insulin resistance, which makes it a perfect option for patients with dermatoses and overweight, such as those with hidradenitis suppurativa.58 However, this needs to be demonstrated in prospective studies with proper follow-up.
Regarding its topical use, roflumilast should be applied once a day, with a small layer being applied to the affected skin. It is not yet available in Spain.
Its application in dermatology parallels former studies on apremilast, another PDE4i with a similar affinity to PDE4 and no higher frequency of side events. However, to date, no studies have compared the side effect profiles of apremilast and roflumilast.79 In our review, we found that roflumilast has been used off-label in its oral form to treat various dermatoses (table 5), including psoriasis, hidradenitis suppurativa,58 recurrent oral aphthosis,59 Behçet's disease,62 erosive oral lichen planus,61 and nummular eczema,60 among others. Also, its pharmacokinetic and pharmacodynamic analogy to apremilast allows us to hypothesize that it could be useful to treat multiple skin diseases. However, the evidence available to date is still limited and recommendations on this regard cannot be made yet. In the coming years, new indications for oral or topical roflumilast in dermatology may be approved, and dermatologists still need to become familiar with this promising drug.
LimitationsThis review has the limitation of being narrative and not a systematic review or meta-analysis. Additionally, many of the studies included, especially those on off-label uses of roflumilast, are case series, have small sample sizes, or a retrospective design. Additionally, its potential uses have been extrapolated from apremilast due to pharmacokinetic and pharmacodynamic analogies. All these factors make it difficult to generalize the findings reported and draw definitive conclusions.
ConclusionsRoflumilast is a drug that has been approved in dermatology to treat plaque psoriasis and mild-to-moderate seborrheic dermatitis, both in topical formulations. There are reports and studies available, most small, which support the utility of oral roflumilast to treat psoriasis, hidradenitis suppurativa, recurrent oral aphthosis, nummular eczema, and lichen planus, among others. It has a very favorable safety profile, is cost-effective, and is widely available. New controlled studies are needed to assess its efficacy profile in inflammatory dermatoses and establish new approved indications for this promising molecule.
Conflicts of interestNone declared.