In the latest edition of its cancer staging manual, the American Joint Committee on Cancer (AJCC) revised the criteria for staging squamous cell carcinoma (SCC) by introducing high-risk tumor features to define tumor stage (T) and help to identify tumors with a higher risk of metastasis. The aim of this study was to investigate the characteristics associated with SCC meeting the high-risk criteria defined by the AJCC for T2 lesions.
Patients and methodWe performed a case-case observational study in which patients with SCC were included over a period of 18 months. We collected clinical, anthropometric, and tumor data, and analyzed these using PASW Statistics (SPSS) version 18.
ResultsOne-hundred eighteen patients, the majority of whom were men, were included. Mean age was 77 years. Over 70% of the tumors were located in the head region and a majority of tumors measured 2cm or less. The prevalence of SCC T2 was 61.9%. The risk factors significantly associated with SCC T2 were an age of over 85 years (odds ratio [OR], 4.48), location in the head and neck region (OR, 3.38), presence of solar elastosis in the peritumoral tissue (OR, 2.08), a higher tumor growth rate (>1.5mm·wk−1; OR, 5.73), and higher cumulative exposure to smoking (>20pack-years, OR, 3.63).
ConclusionsAdvanced age, location in the head and neck region, presence of solar elastosis, high tumor growth rate, and high cumulative smoking exposure were all significantly associated with the presence of SCC T2.
En la última edición del manual de la American Joint Committee on Cancer (AJCC) se modificó la estadificación para el carcinoma epidermoide cutáneo (CEC), introduciendo características tumorales de alto riesgo que definen el estadio tumoral (T), con el propósito de identificar aquellos tumores con mayor riesgo de metástasis. Nuestro objetivo fue definir las características asociadas al CEC que cumplía criterios de alto riesgo definidos por la AJCC para ser estadio T2.
Pacientes y métodoEstudio observacional analítico tipo casos-casos de 18 meses donde se han incluido pacientes con diagnóstico de CEC. Se recogieron datos clínicos, antropométricos y tumorales. Para el análisis estadístico se ha utilizado la versión 18.0 del programa PASW Statistics (SPSS).
ResultadosEl número total de pacientes incluidos fue 118. La edad media de la población fue de 77 años, con predominio del sexo masculino. Más del 70% de los CEC se presentaron en la región cefálica, y la mayoría fue ≤2cm. La prevalencia de CEC T2 fue del 61,9%. Los factores de riesgo estadísticamente significativos asociados al CEC estadio T2 fueron: la edad (>85 años, OR: 4,48), la localización en la cabeza y el cuello (OR 3,38), la presencia de elastosis solar en el tejido peritumoral (OR 2,08), la tasa de crecimiento más elevada (>1,5 mm*sem-1, OR: 5,73) y el grupo de mayor exposición tabáquica (>20 años/paquete; OR: 3,63).
ConclusionesLa edad avanzada, la localización en la cabeza y el cuello, la presencia de elastosis solar, la velocidad de crecimiento más elevada y la exposición tabáquica intensa son los factores de riesgo que se asociaron a la presencia de CEC estadio T2.
In light-skinned populations, nonmelanoma skin tumors comprise the most prevalent type of cancer and their incidence is rising all over the world.1–4 Rates of squamous cell carcinoma (SCC) in particular have increased markedly in recent decades, by 3% to 10% per year according to some studies.1–4 In Spain, the incidence of SCC is 72 per 100000 person-years for females and 100 per 100000 person-years for males.5 SCC tumors arise in epidermal keratinocytes. The etiology is multifactorial, but the most important factor is exposure to UV radiation (from sunlight, psoralen-UV-A therapy, or tanning booths).6 Other relevant factors that favor the development of SCC are exposure to ionizing radiation7; contact with chemicals such as pesticides, tar, polycyclic aromatic hydrocarbons; and exposure to arsenic.8 Immunocompromised patients also have higher rates of SCC, and their tumors behave more aggressively and have a greater tendency to metastasize than tumors in the general population. Recipients of solid-organ transplants (such as a heart or kidney) are especially at risk. Human papilloma virus infection has been linked to the pathogenesis of SCC; particularly implicated are Betapapillomavirus species, which infect nonmucosal cutaneous tissues and are more prevalent in immunocompromised patients.9–11 Prior history of an inflammatory lesion, dermatitis, long-established wounds or ulcers, or another skin tumor are also considered risk factors for SCC.
The seventh (2010) edition of the staging manual of the American Joint Committee on Cancer (AJCC) introduced important new instructions for SCC staging in the interest of offering more useful prognostic information and therefore providing better guidance on treatment.12–14 One important change was that SCC was distinguished from the set of other nonmelanoma skin cancers such as basal cell and Merkel cell carcinomas. Under the new system, other important factors in addition to tumor size (>2cm) are taken into account. Examples are depth over 2mm and the extent of deep structure involvement (Clark level ≥4), location in a high-risk site (ear or lip), or grade (poorly differentiated). If a tumor meets 2 or more of these high-risk criteria it is shifted to a higher T class than would apply based on size alone, and the prognosis would therefore be worse. However, even though prognosis is more accurate under this staging system, other predictors of high risk that are associated with higher rates of recurrence and metastasis are not included; examples are the presence of lymphovascular invasion, immunosuppression, history of a lesion in the same area, and location in high-risk sites other than the ear or lip.15,16
Our study aimed to determine the prevalence of SCC meeting these AJCC criteria for high risk in a patient series and to explore associated factors.
Patients and MethodsThis observational study analyzed data gathered for cases and controls. After our hospital's ethics committee approved the study in July 2012, recruitment took place in the dermatology department over a period of 18 months.
The inclusion criteria were as follows: diagnosis of SCC, age over 18 years, written informed consent, and under treatment by physicians in our department in a Mediterranean coastal area with 379000 inhabitants in eastern Spain.
Patients with SCC were excluded if they declined to participate, died before treatment of the tumor, or had a firm or suspected diagnosis of any genetic condition linked to skin cancer. Samples with flaws that could not be processed, and genital mucosal SCCs were also excluded.
The following information was recorded during the first visit or taken from the patient's chart if available.
Clinical Characteristics- -
Sex and age
- -
Regular sun exposure, considering years of regular occupational or recreational exposure, on 3 levels:
Low: occasional, exposure that is mainly recreational and not prolonged
Moderate: occupational exposure of less than 15 years’ duration or recreational exposure of less than 3hours per day, mainly in the summer
High: occupational exposure longer than 15 years’ duration or summer recreational exposure averaging over 3hours per day or maintained for long periods
- -
Phototype I-VI (Fitzpatrick scale)
- -
Smoking, classified according to the instructions of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) for calculating pack-years of exposure as follows: number of cigarettes per day, multiplied by years of smoking, divided by 20. We considered that fewer than 9 pack-years indicated light smoking exposure; between 10 and 20, moderate exposure, and more than 20, high exposure.
- -
Alcohol (units/day) and drug use (number of drugs and type)
- -
Body mass index (kg/m2)
- -
Chronic comorbidity (e.g., dyslipidemia, diabetes mellitus, hypertension, inflammatory disorders)
- -
Primary or secondary immunosuppression: blood disorders, immunosuppressant treatment, human immunodeficiency virus infection, transplantation, etc.
- -
History of dermatologic disease: actinic keratosis, SCC in situ (Bowen disease), nonmelanoma skin cancer
- -
Exposure to carcinogens such as arsenic, coal tar products, ionizing radiation
- -
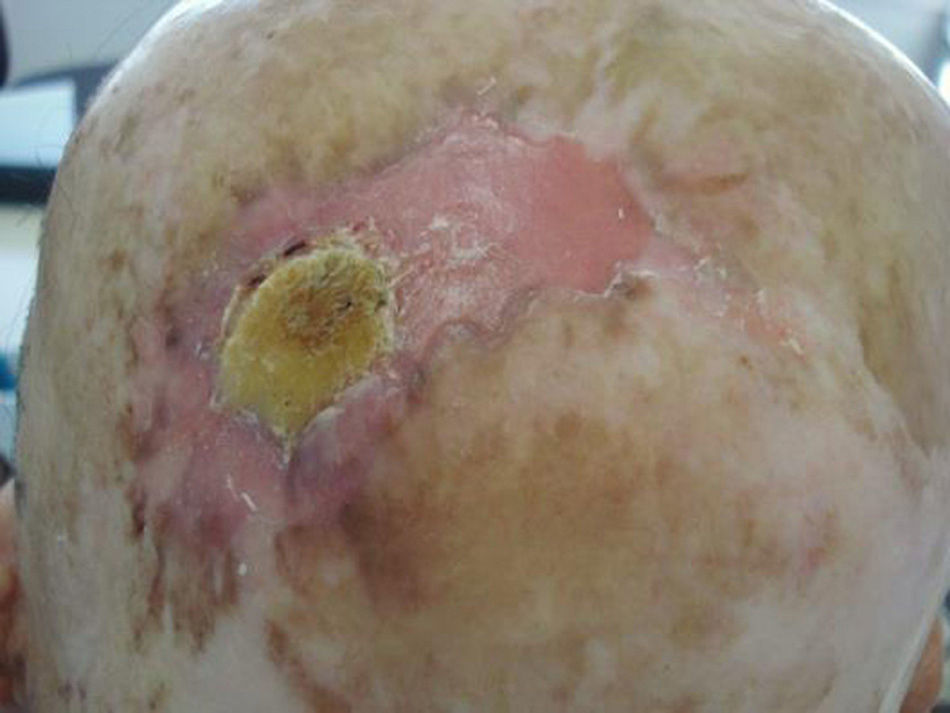
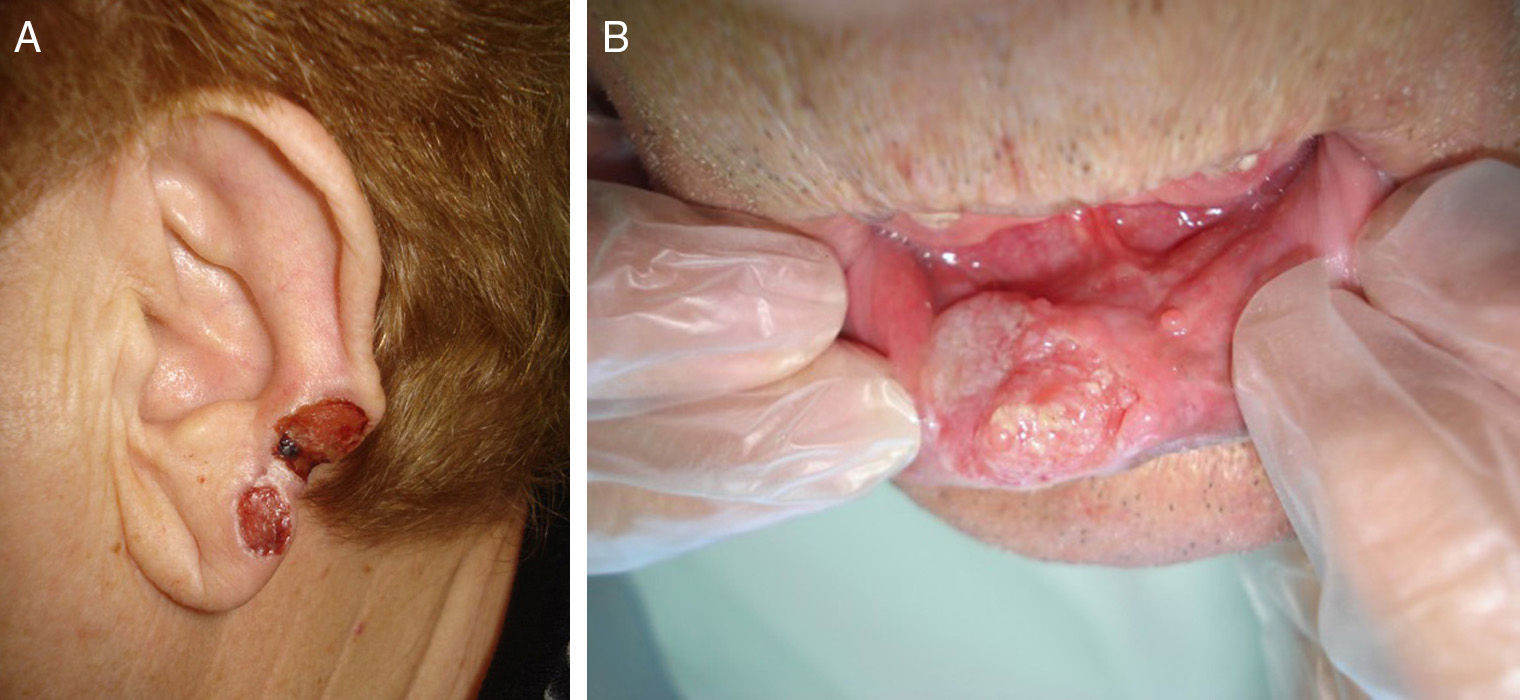
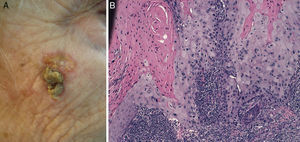
Prior lesions in the region of the tumor (e.g., chronic ulcers or burns) (Fig. 1), actinic keratosis, or nonmelanoma skin cancer
- -
Time in months since the patient noticed the lesion until it was excised
- -
Tumor location: center of the face, ear, lips, scalp, back, chest, abdomen, arms, hands, legs, or feet. For descriptive statistics we grouped tumors by regions considered to confer high risk according to the AJCC manual. In the multivariate analysis head-and-neck tumors were compared to any other location.
Histologic features recorded by the pathologists were analyzed. The protocol of the seventh edition of the AJCC manual was used to classify tumors. Data collected were as follows:
- -
Tumor size in centimeters
- -
Tumor depth (Clark level and Breslow depth in millimeters)
- -
Presence or not of perineural or lymphovascular invasion
- -
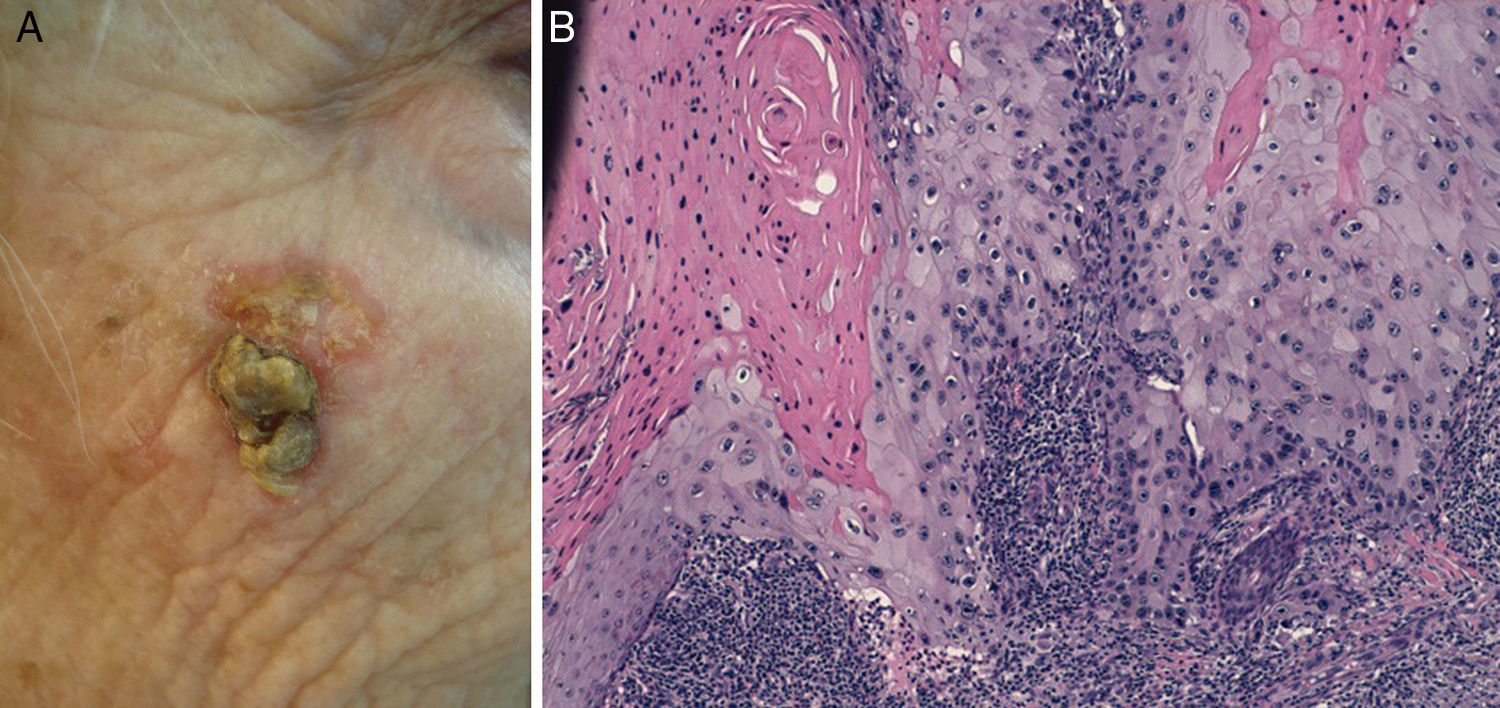
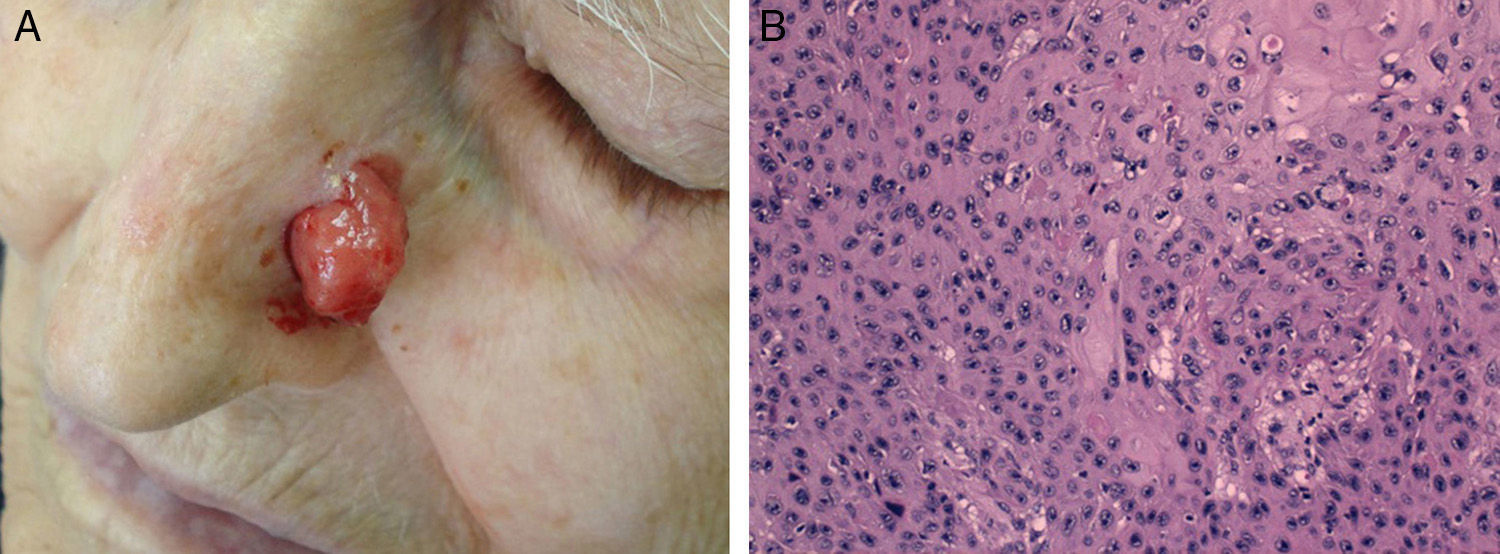
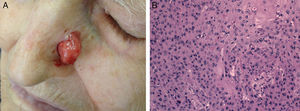
Cell differentiation (Figs. 2 and 3): well differentiated, moderately differentiated, or poorly differentiated
- -
Mitotic rate (mitoses/mm2), necrosis, or increased vascularity
- -
Histologic subtype: keratinizing, nodular, ulcerated, verrucous, flat, clear-cell, mixed (if several features were present in the same tumor), and other (any type not on this list)
- -
State of surrounding tissues: solar elastosis, in situ SCC, scarring, actinic keratosis
- -
Tumor-free margins (lateral and deep) after excision, in millimeters
We also analyzed growth rate (in millimeters per week) calculated as tumor thickness or Breslow depth (in millimeters) divided by the time difference (in weeks) between the moment the patient noticed the lesion and the moment of excision.
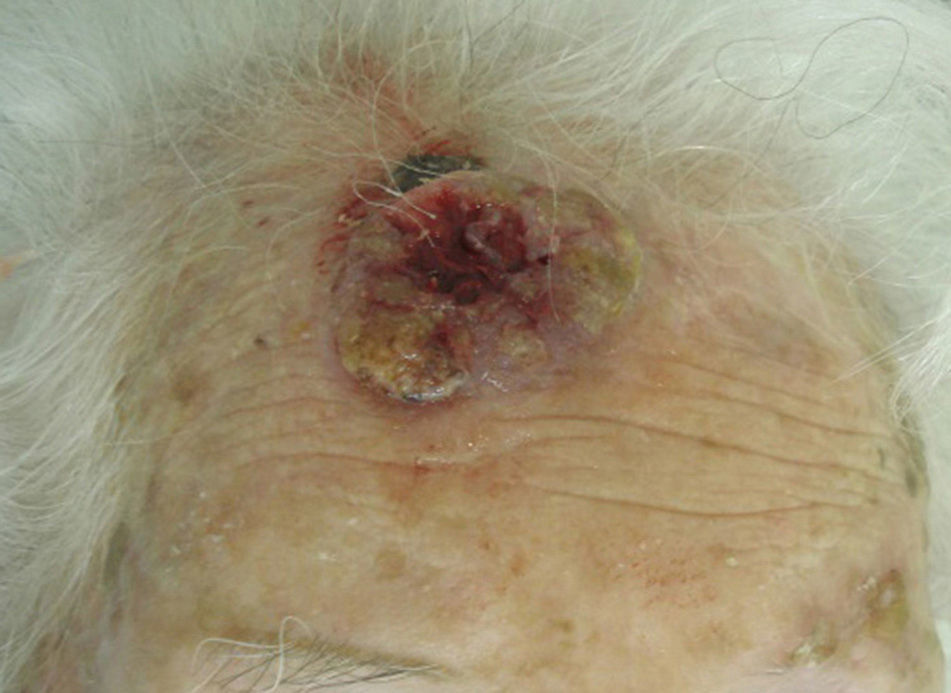
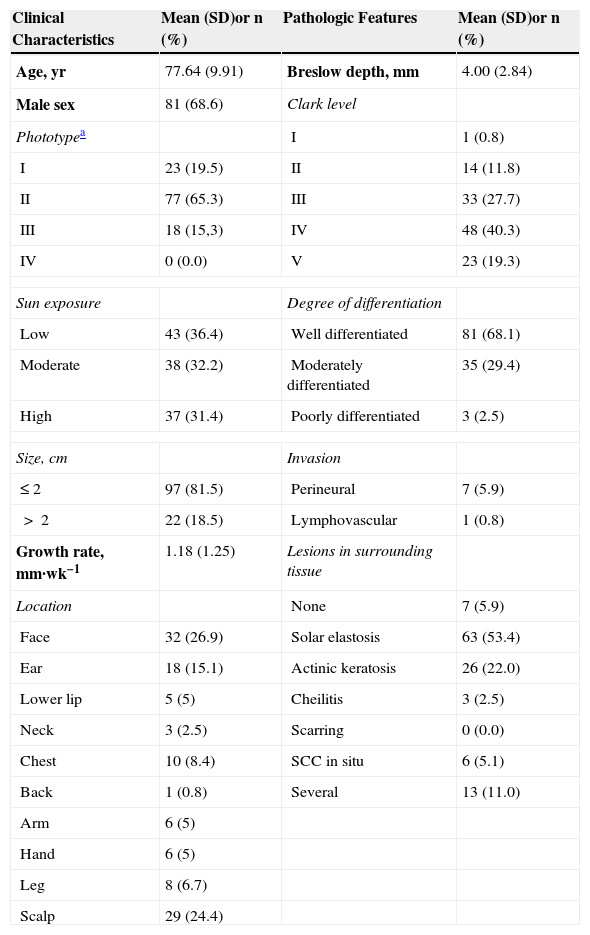
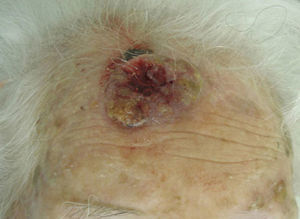
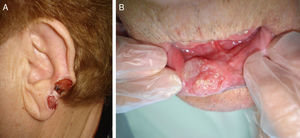
The classification of SCC tumors as having a worse prognosis (T2) according to the characteristics outlined in the seventh edition of the AJCC manual13 was first based on size (>2cm) (Fig. 4). Additionally, smaller tumors were also classified T2 if they had at least 2 of the following characteristics: location in a high-risk site (ear or lips) (Fig. 5), depth of more than 2mm (or Clark level≥4), or poor differentiation.
Quantitative data were analyzed for normality of distribution with the Kolmogorov-Smirnov test. Normally distributed data were described by the mean (SD) and the 95% CI. Nonnormally distributed data were described by the median (interquartile range). Qualitative values were described by percentages.
The χ2 test was used for between-group comparisons of dichotomous variables expressed as percentages, and the t test was used to compare continuous variables. Pearson's correlation coefficients were calculated to describe associations between each independent variable and the dependent variable. Multivariate regression analysis was then used to confirm the independence of the factors found to correlate with high-risk SCC classification. Finally, logistic regression analysis was used to estimate adjusted and crude odds ratios (OR) and 95% CIs to clarify which factors predicted that an SCC would meet the criteria for high risk (T2 classification) according to the seventh AJCC staging manual. Cut points were calculated based on normal values for control patients. Data were expressed as mean (SD).
A P value <.05 was considered statistically significant in 2-tailed comparisons.
ResultsData from records for 118 patients were studied. Eighty-one (68.8%) were men. The mean patient age was 77.6 years (median [interquartile range], 79[13]). More than half (77,65.3%) had phototype II skin, and 23(19.5%) had phototypeI skin. Similar numbers of patients fell into each of the 3 sun-exposure groups: 43(36.4%) reported a low level of exposure, 38(32.2%) a moderate level, and 37(31.4%) a high level.
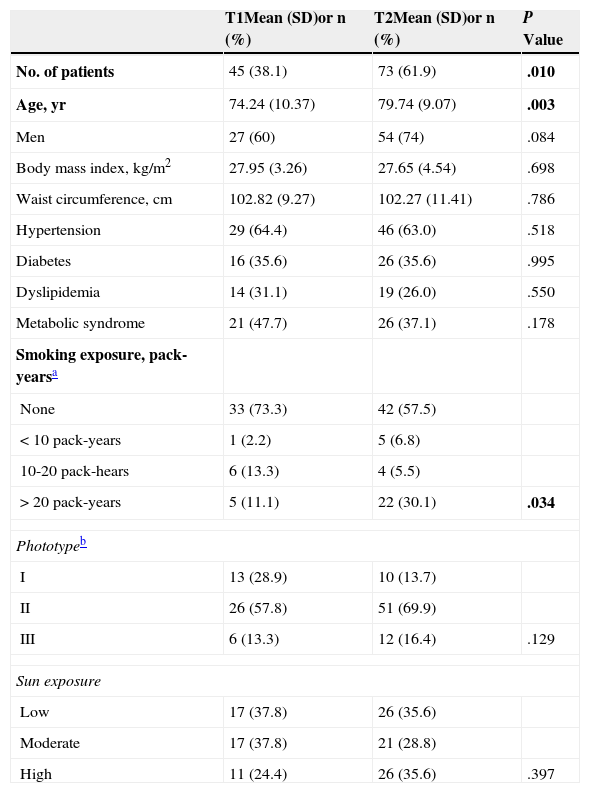
Most patients (97, 81.5%) had SCCs of 2cm or less; the mean growth rate was 1.18mm/wk. The most common locations were the face (32,26.9%) and scalp (29,24.4%), followed by the ear (18,15.1%). Over 70% of head tumors were in these locations. Keratinizing tumors (50,42.4%) comprised the most common morphologic type. Nodular tumors were the next-largest group (25,21.2%), followed by ulcerated tumors (19,9.3%). The mean Breslow depth, indicating the maximum penetration, was 4(2.84)mm, and nearly 60%(71) had invaded the reticular dermis or subcutaneous tissue (Clark levels 4 and 5, respectively). These characteristics are summarized in Table 1.
Demographic and Clinical Characteristics of the Studied Population of 118 Patients With Squamous Cell Carcinomas and the Pathologic Features of the Tumors.
| Clinical Characteristics | Mean (SD)or n (%) | Pathologic Features | Mean (SD)or n (%) |
|---|---|---|---|
| Age, yr | 77.64 (9.91) | Breslow depth, mm | 4.00 (2.84) |
| Male sex | 81 (68.6) | Clark level | |
| Phototypea | I | 1 (0.8) | |
| I | 23 (19.5) | II | 14 (11.8) |
| II | 77 (65.3) | III | 33 (27.7) |
| III | 18 (15,3) | IV | 48 (40.3) |
| IV | 0 (0.0) | V | 23 (19.3) |
| Sun exposure | Degree of differentiation | ||
| Low | 43 (36.4) | Well differentiated | 81 (68.1) |
| Moderate | 38 (32.2) | Moderately differentiated | 35 (29.4) |
| High | 37 (31.4) | Poorly differentiated | 3 (2.5) |
| Size, cm | Invasion | ||
| ≤2 | 97 (81.5) | Perineural | 7 (5.9) |
| >2 | 22 (18.5) | Lymphovascular | 1 (0.8) |
| Growth rate, mm·wk−1 | 1.18 (1.25) | Lesions in surrounding tissue | |
| Location | None | 7 (5.9) | |
| Face | 32 (26.9) | Solar elastosis | 63 (53.4) |
| Ear | 18 (15.1) | Actinic keratosis | 26 (22.0) |
| Lower lip | 5 (5) | Cheilitis | 3 (2.5) |
| Neck | 3 (2.5) | Scarring | 0 (0.0) |
| Chest | 10 (8.4) | SCC in situ | 6 (5.1) |
| Back | 1 (0.8) | Several | 13 (11.0) |
| Arm | 6 (5) | ||
| Hand | 6 (5) | ||
| Leg | 8 (6.7) | ||
| Scalp | 29 (24.4) | ||
Abbreviation: SCC, squamous cell carcinoma.
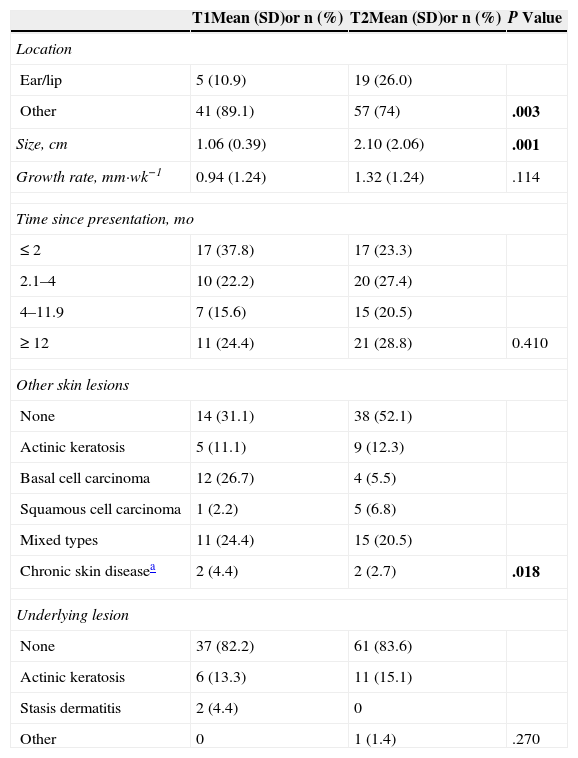
Table 2 compares patient characteristics, medical histories, and substance use between patients with tumors with a better prognosis (T1 classification) or a worse prognosis (T2). Patients with T2 SCCs were older. Men were more numerous than women in both groups, but the proportion of men was significantly higher in the T2 group (P=.084). The T2 group also had more smokers, who also had much higher levels of smoking exposure (3-fold higher proportion of smokers with >20 pack-years) than the smokers in the T1 group.
Comparison of Demographic and Anthropometric Characteristics, Comorbidity, and Smoking of Patients With T1 and T2 Squamous Cell Carcinomas.
| T1Mean (SD)or n (%) | T2Mean (SD)or n (%) | P Value | |
|---|---|---|---|
| No. of patients | 45 (38.1) | 73 (61.9) | .010 |
| Age, yr | 74.24 (10.37) | 79.74 (9.07) | .003 |
| Men | 27 (60) | 54 (74) | .084 |
| Body mass index, kg/m2 | 27.95 (3.26) | 27.65 (4.54) | .698 |
| Waist circumference, cm | 102.82 (9.27) | 102.27 (11.41) | .786 |
| Hypertension | 29 (64.4) | 46 (63.0) | .518 |
| Diabetes | 16 (35.6) | 26 (35.6) | .995 |
| Dyslipidemia | 14 (31.1) | 19 (26.0) | .550 |
| Metabolic syndrome | 21 (47.7) | 26 (37.1) | .178 |
| Smoking exposure, pack-yearsa | |||
| None | 33 (73.3) | 42 (57.5) | |
| <10 pack-years | 1 (2.2) | 5 (6.8) | |
| 10-20 pack-hears | 6 (13.3) | 4 (5.5) | |
| >20 pack-years | 5 (11.1) | 22 (30.1) | .034 |
| Phototypeb | |||
| I | 13 (28.9) | 10 (13.7) | |
| II | 26 (57.8) | 51 (69.9) | |
| III | 6 (13.3) | 12 (16.4) | .129 |
| Sun exposure | |||
| Low | 17 (37.8) | 26 (35.6) | |
| Moderate | 17 (37.8) | 21 (28.8) | |
| High | 11 (24.4) | 26 (35.6) | .397 |
The average tumor growth rate was also higher in patients with class T2 tumors (P=.114) (Table 3). The remaining clinical characteristics and pathologic features are summarized in Tables 3 and 4.
Comparison of the Clinical Characteristics of Squamous Cell Carcinomas According to Lower (T1) or Higher (T2) Risk Classification.
| T1Mean (SD)or n (%) | T2Mean (SD)or n (%) | P Value | |
|---|---|---|---|
| Location | |||
| Ear/lip | 5 (10.9) | 19 (26.0) | |
| Other | 41 (89.1) | 57 (74) | .003 |
| Size, cm | 1.06 (0.39) | 2.10 (2.06) | .001 |
| Growth rate, mm·wk−1 | 0.94 (1.24) | 1.32 (1.24) | .114 |
| Time since presentation, mo | |||
| ≤2 | 17 (37.8) | 17 (23.3) | |
| 2.1–4 | 10 (22.2) | 20 (27.4) | |
| 4–11.9 | 7 (15.6) | 15 (20.5) | |
| ≥12 | 11 (24.4) | 21 (28.8) | 0.410 |
| Other skin lesions | |||
| None | 14 (31.1) | 38 (52.1) | |
| Actinic keratosis | 5 (11.1) | 9 (12.3) | |
| Basal cell carcinoma | 12 (26.7) | 4 (5.5) | |
| Squamous cell carcinoma | 1 (2.2) | 5 (6.8) | |
| Mixed types | 11 (24.4) | 15 (20.5) | |
| Chronic skin diseasea | 2 (4.4) | 2 (2.7) | .018 |
| Underlying lesion | |||
| None | 37 (82.2) | 61 (83.6) | |
| Actinic keratosis | 6 (13.3) | 11 (15.1) | |
| Stasis dermatitis | 2 (4.4) | 0 | |
| Other | 0 | 1 (1.4) | .270 |
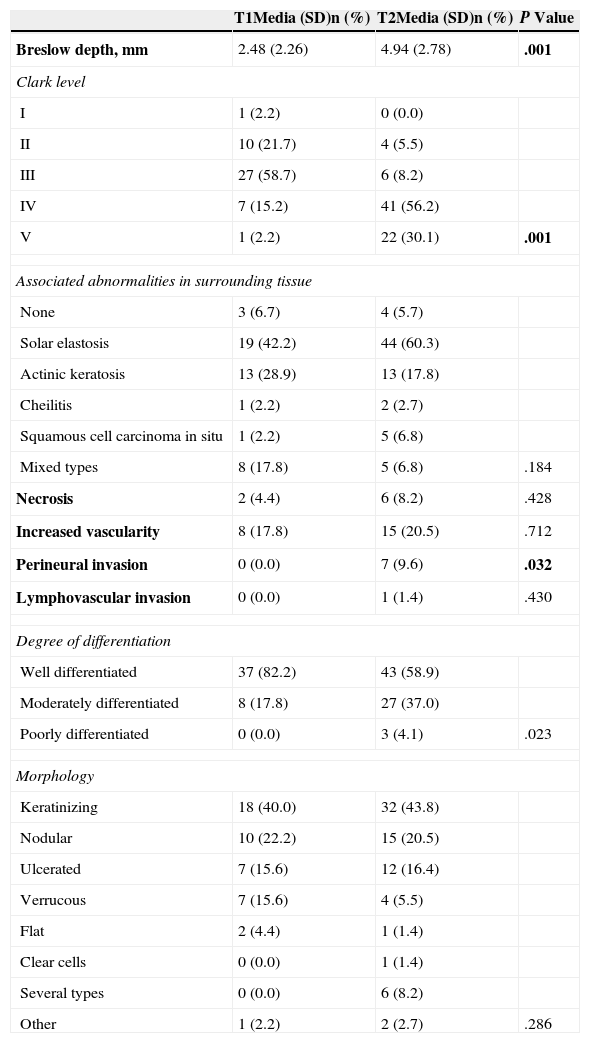
Comparison of Pathologic Features of T1 and T2 Squamous Cell Carcinomas.
| T1Media (SD)n (%) | T2Media (SD)n (%) | P Value | |
|---|---|---|---|
| Breslow depth, mm | 2.48 (2.26) | 4.94 (2.78) | .001 |
| Clark level | |||
| I | 1 (2.2) | 0 (0.0) | |
| II | 10 (21.7) | 4 (5.5) | |
| III | 27 (58.7) | 6 (8.2) | |
| IV | 7 (15.2) | 41 (56.2) | |
| V | 1 (2.2) | 22 (30.1) | .001 |
| Associated abnormalities in surrounding tissue | |||
| None | 3 (6.7) | 4 (5.7) | |
| Solar elastosis | 19 (42.2) | 44 (60.3) | |
| Actinic keratosis | 13 (28.9) | 13 (17.8) | |
| Cheilitis | 1 (2.2) | 2 (2.7) | |
| Squamous cell carcinoma in situ | 1 (2.2) | 5 (6.8) | |
| Mixed types | 8 (17.8) | 5 (6.8) | .184 |
| Necrosis | 2 (4.4) | 6 (8.2) | .428 |
| Increased vascularity | 8 (17.8) | 15 (20.5) | .712 |
| Perineural invasion | 0 (0.0) | 7 (9.6) | .032 |
| Lymphovascular invasion | 0 (0.0) | 1 (1.4) | .430 |
| Degree of differentiation | |||
| Well differentiated | 37 (82.2) | 43 (58.9) | |
| Moderately differentiated | 8 (17.8) | 27 (37.0) | |
| Poorly differentiated | 0 (0.0) | 3 (4.1) | .023 |
| Morphology | |||
| Keratinizing | 18 (40.0) | 32 (43.8) | |
| Nodular | 10 (22.2) | 15 (20.5) | |
| Ulcerated | 7 (15.6) | 12 (16.4) | |
| Verrucous | 7 (15.6) | 4 (5.5) | |
| Flat | 2 (4.4) | 1 (1.4) | |
| Clear cells | 0 (0.0) | 1 (1.4) | |
| Several types | 0 (0.0) | 6 (8.2) | |
| Other | 1 (2.2) | 2 (2.7) | .286 |
Bold face type marks statistically significant findings.
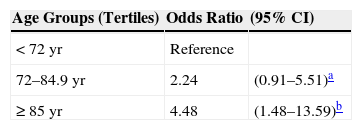
Tables 5 and 6 show the results of initial and multivariate risk analysis. Age was associated with developing a class T2 SCC, and risk increased by age tertile (Table 5). Risk was 4-fold higher in the oldest group of patients (≥85years) (OR, 4.48; 95%CI,1.48–13.59).
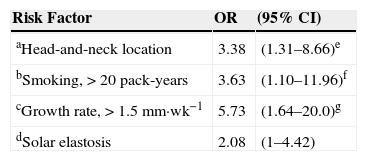
Risk Factors for Developing T2 Squamous Cell Carcinoma: Odds Ratios Adjusted for Age, Sex, Body Mass Index, and Sun Exposure.
| Risk Factor | OR | (95% CI) |
|---|---|---|
| aHead-and-neck location | 3.38 | (1.31–8.66)e |
| bSmoking, >20 pack-years | 3.63 | (1.10–11.96)f |
| cGrowth rate, >1.5mm·wk−1 | 5.73 | (1.64–20.0)g |
| dSolar elastosis | 2.08 | (1–4.42) |
aVs. other sites.
bSmokers vs. nonsmokers.
cFaster- vs. slower-growing tumors.
dIn peritumoral tissue.
eP=.011.
fP=.034.
gP=.006.
The presence of peritumoral solar elastosis was also associated with T2 SCC classification (OR,2.08), as was location on the head or neck (4.12 times more frequent; OR,3.38 when adjusted for age, sex, body mass index, and sun exposure) (Table 6). We also calculated the OR for head-and-neck SCCs adjusted for the same variables but eliminating SCCs on the lip and ear to correct for selection bias; that analysis confirmed that the risk that a head-and-neck location would indicate worse prognosis (OR,2.90; 95%CI,1.12–7.49).
Higher cumulative smoking exposure (>20 pack-years) also indicated higher risk (OR,3.42) of T2 classifiction, and risk remained high when adjusted for age and sex (OR,3.63) (Table 6). When lower lip SCCs were removed from the analysis to further correct for selection bias, given that smoking is known to predict SCC in oral mucosa, the OR increased to 4.04(95%CI,1.11–14.7).
Risk for T2 classification rose sharply for SCCs with higher growth rates (OR,4.58 in comparison with slower growing tumors) and when adjusted for age and sex the OR increased further to 5.73.
DiscussionAdvanced age, head-and-neck location, rapid growth rate, and higher cumulative smoking exposure were the independent risk factors associated with T2 classification in this study of SCCs.
The prevalence of SCC meeting the criteria for T2 classification according to the seventh edition of the AJCC manual12,13 was 61.9%. A study of 257 SCCs also analyzed according to these AJCC criteria at a university medical center in the US city of St Louis found a much lower rate (13.9%) of T2 tumors.17
A finding we have not seen reported in the literature to date is the association we observed between T2 classification and the presence of solar elastosis in tissue surrounding the SCC, although this association has been reported for melanoma.18 In our study, this characteristic was associated with T2 classification, but as the grading of solar elastosis had not been specified in the pathology protocol, it could not be correlated with other variables, such as degree of sun exposure or tumor location.19
Advanced age was associated with T2 classification in this series of patients with SCC, in which the average patient age was around 79 years, and the group with lowest risk, or most likely to have T1 SCCs, were those aged under 74 years. In fact, on stratifying our patients into 3 age groups, risk of T2 classification in the oldest tertile (>85 years of age) was 4-fold greater than in the tertile of patients aged under 72 years. As age is associated with greater immune compromise, older patients are more likely to develop more aggressive tumors, an interpretation consistent with the findings of Corbalán-Vélez et al.19
How SCC location is related to higher risk of poor prognosis is currently much discussed; the only sites the AJCC considers high risk are the lip and ear, whereas other expert committees place all head-and-neck tumors in this risk category.20 In fact there are multiple publications reporting data on prognosis and survival that focus only on SCCs in this region.21–26 T2 classification remained strongly associated with a head-and-neck location in our series, even after we adjusted for possible confounders (such as tumors on the lip or ear).
SCCs with the highest growth rate of over 1.5 mm·wk−1 were 4-fold more likely to be classified as T2 tumors. This finding is consistent with data presented by Martorell et al.27 at the Spanish national dermatology society's 42nd National Conference of Dermatology and Venereology. Those authors observed that SCCs with growth rates of 5mm/mo were high-risk variants. Our results were very similar: high-risk was associated with a growth rate over 6mm/mo, even when we adjusted for other characteristics when looking at the association.
Our data confirmed that smoking is related to T2 SCC classification. Although smoking has traditionally been linked to higher incidences of SCC, as we mentioned in our introduction, cohort studies published in recent years28,29 have been unable to find an association between SCC and smoking intensity or duration; nor has system of nicotine delivery been associated with risk. In our study, however, T2 SCC classification was associated with higher cumulative exposure (>20pack-years), as confirmed by multivariate analysis.
We found no associations between the presence of high-risk SCC classification and degree of sun exposure, sex, skin phototype, or the presence of such concomitant conditions as hypertension, diabetes mellitus, dyslipidemia, or metabolic syndrome; we think this lack of association suggests that these characteristics do not influence prognosis in SCC.
Given SCC's high prevalence and associated complications, and the consequent public health importance of this disease, patients whose tumors can be predicted to behave more aggressively should be identified so that optimal management and careful follow-up can be implemented. Few studies like this one, aiming to define the relevant characteristics and risk factors associated with aggressive SCCs, have been done to date.
The limitations of our study are those inherent to any observational analysis: assessments of patient exposures and relevant events took place at specific moments, and some exposures or other assessments—e.g., smoking, sun exposure, time the lesion was first noticed—were estimated by the patients themselves. A degree of subjective judgement, therefore, was involved.
Because of the high prevalence of SCCs meeting the seventh-edition AJCC criteria for T2 (high-risk) staging, we wonder whether these criteria might not be overly broad. Therefore, we think that related risk factors should be explored further and that cohort studies should be undertaken to provide information on medium- to long-term survival and metastatic activity in SCC.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for the purpose of this study.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in this article. The signed forms are in the possession of the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Díaz-Corpas T, Morales-Suárez-Varela M, Rausell Fontestad N, Fuertes Prósper A, Marquina-Vila A, Jordá-Cuevas E. Carcinoma epidermoide cutáneo: definición de sus características clínico-patológicas y factores de riesgo asociados en un estudio observacional de 118 pacientes. Actas Dermosifiliogr. 2015;106:806–815.