Patients with chronic inflammatory diseases being treated with immunosuppressive drugs, and with tumor necrosis factor inhibitors in particular, have an increased risk of infection by Mycobacterium tuberculosis. Screening for latent tuberculosis infection and preventive therapy to reduce the risk of progression to active tuberculosis are mandatory in this group of patients. This updated multidisciplinary consensus document presents the latest expert opinions on the treatment and prevention of tuberculosis in candidates for biologic therapy and establishes recommendations based on current knowledge relating to the use of biologic agents.
El riesgo de infección por Mycobacterium tuberculosis se halla aumentado en los pacientes con enfermedades inflamatorias crónicas y en tratamiento inmunosupresor, en particular con terapia antifactor de necrosis tumoral α. La detección de la infección tuberculosa latente y el tratamiento preventivo dirigido a reducir el riesgo de progresión a tuberculosis activa es obligatoria en este grupo de pacientes. Este documento de consenso multidisciplinar actualiza la opinión de expertos y establece recomendaciones para el diagnóstico y tratamiento de la infección tuberculosa latente en estos pacientes, según los conocimientos actuales en terapias biológicas.
According to current estimates, a third of the world population is a carrier of latent Mycobacterium tuberculosis infection,1 which can become reactivated, particularly in immunocompromised patients. The estimated incidence of latent tuberculosis infection (LTI) in Spain in patients with psoriasis treated with anti-tumor necrosis factor (TNF) agents is 29%.2 In the best case scenario, only 10% of those infected will develop active tuberculosis (TB) during their lifetimes.3,4 However, there are factors that may significantly increase this percentage and so latent infection is considered a major obstacle for worldwide elimination of TB. Public health measures and, ultimately, clinical management of TB infection could be improved if we had a better knowledge of latent and reactivated states of M tuberculosis.
On the other hand, in the past 10 years, development of biological therapies as led to a major change in the treatment of some chronic inflammatory skin diseases, and psoriasis in particular, but also hidradenitis suppurativa after the recent approval of adalimumab in that indication. In 1998, the Food and Drug Administration approved the use of infliximab in patients with psoriasis who are refractory to conventional immunomodulatory treatment.5 Since then, more than 20 new drugs have become available for the treatment of immune-mediated inflammatory diseases (IMIDs) in which TNF and its receptors play a key role in acute and chronic inflammatory responses.6
TNF is important for immune response, and so drugs that target this molecule may increase the risk of infections and TB reactivation.
Postmarketing surveillance of the first biological agents to be approved (infliximab and etanercept) soon revealed the appearance of associated cases of TB.7 In Spain, since February 2000, a surveillance program with active data acquisition in the Biological Products Database of the Spanish Rheumatology Society (BIOBADASER)8 has been implemented and in October 2008, the Spanish registry of systemic psoriasis treatments (BIOBADADERM) was set up as part of the pharmacovigilance strategy.9
Evidence from reported cases showed a strong association between infliximab therapy and increased risk of active TB. This soon forced guidelines and recommendations to be established such as the one published in 2003 by the Spanish Work Group for Crohn Disease and Ulcerative Colitis (GETECCU),10 for the prevention of TB in candidates for infliximab treatment.
Diagnosis of LTI and preventive treatment with isoniazid (INH) for 9 months has been established as a means to reduce the probability of progression to active TB,11,12 and it has been shown that implementation of LTI screening protocols in candidates for anti-TNF therapy has reduced the incidence of TB by more than 78%.13 However, new cases have continued to arise, even after preventive treatment with INH,14 and this has forced the protocols to be updated and attempts to improve the sensitivity and specificity of the diagnostic tests and treatments in this population, with a high risk of progression to active TB.15
Objectives of the DocumentThe availability of abundant and recent information on new biological therapies and the lack of guidelines agreed among different Spanish scientific societies was the rationale behind the need for a consensus document, published in 2016. This document aimed to collate existing information and recommendations on the basis of evidence available and consensus of a group of experts.16 The objective of the present document is to update and extend the information in the earlier document and adapt it to dermatology patients.
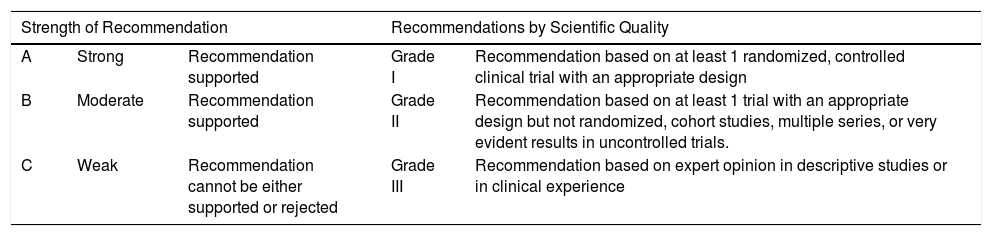
MethodologyThe document has been drafted by designated experts from the participating societies (Spanish Academy of Dermatology and Venereology, Spanish Society of Pneumology and Thoracic Surgery, Spanish Society of Digestive Pathology, Spanish Society of Rheumatology, and Spanish Society of Infectious Diseases and Clinical Microbiology) who spend a large part of their time on the study and control of candidates for biological therapies and who have particular experience in this field. The most recent editions of Spanish and international guidelines for biological diseases, diagnosis and treatment of LTI, and databases of Medline and Cochrane up until July 2017 were consulted. With the information obtained, recommendations were established based on the classification of the Infectious Diseases Society of America,17 as well as the strength of the recommendation18 (Table 1).
Degree of Recommendation18
| Strength of Recommendation | Recommendations by Scientific Quality | |||
|---|---|---|---|---|
| A | Strong | Recommendation supported | Grade I | Recommendation based on at least 1 randomized, controlled clinical trial with an appropriate design |
| B | Moderate | Recommendation supported | Grade II | Recommendation based on at least 1 trial with an appropriate design but not randomized, cohort studies, multiple series, or very evident results in uncontrolled trials. |
| C | Weak | Recommendation cannot be either supported or rejected | Grade III | Recommendation based on expert opinion in descriptive studies or in clinical experience |
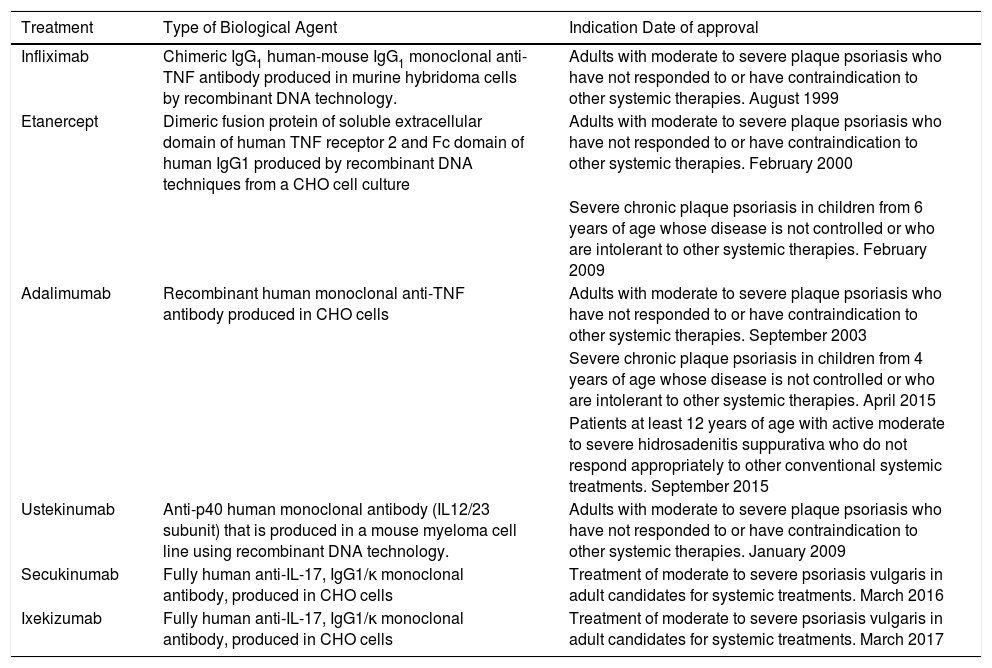
Currently, the biological agents with an indication approved in Spain for psoriasis are infliximab, etanercept, adalimumab, ustekinumab, secukinumab, and ixekizumab.19–31 Adalimumab has also recently been approved for the treatment of hidrosadenitis suppurativa.32 There are no other biological agents approved for diseases specifically of the skin. Table 2 summarizes the main indications for each biological agent and the date of approval in Spain.19–24
Type of Biological Agent, Indication, and Date of Approval.
| Treatment | Type of Biological Agent | Indication Date of approval |
|---|---|---|
| Infliximab | Chimeric IgG1 human-mouse IgG1 monoclonal anti-TNF antibody produced in murine hybridoma cells by recombinant DNA technology. | Adults with moderate to severe plaque psoriasis who have not responded to or have contraindication to other systemic therapies. August 1999 |
| Etanercept | Dimeric fusion protein of soluble extracellular domain of human TNF receptor 2 and Fc domain of human IgG1 produced by recombinant DNA techniques from a CHO cell culture | Adults with moderate to severe plaque psoriasis who have not responded to or have contraindication to other systemic therapies. February 2000 |
| Severe chronic plaque psoriasis in children from 6 years of age whose disease is not controlled or who are intolerant to other systemic therapies. February 2009 | ||
| Adalimumab | Recombinant human monoclonal anti-TNF antibody produced in CHO cells | Adults with moderate to severe plaque psoriasis who have not responded to or have contraindication to other systemic therapies. September 2003 |
| Severe chronic plaque psoriasis in children from 4 years of age whose disease is not controlled or who are intolerant to other systemic therapies. April 2015 | ||
| Patients at least 12 years of age with active moderate to severe hidrosadenitis suppurativa who do not respond appropriately to other conventional systemic treatments. September 2015 | ||
| Ustekinumab | Anti-p40 human monoclonal antibody (IL12/23 subunit) that is produced in a mouse myeloma cell line using recombinant DNA technology. | Adults with moderate to severe plaque psoriasis who have not responded to or have contraindication to other systemic therapies. January 2009 |
| Secukinumab | Fully human anti-IL-17, IgG1/κ monoclonal antibody, produced in CHO cells | Treatment of moderate to severe psoriasis vulgaris in adult candidates for systemic treatments. March 2016 |
| Ixekizumab | Fully human anti-IL-17, IgG1/κ monoclonal antibody, produced in CHO cells | Treatment of moderate to severe psoriasis vulgaris in adult candidates for systemic treatments. March 2017 |
Abbreviations; CHO, Chinese hamster ovary; TNF, tumor necrosis factor.
Currently, new therapeutic options are emerging for the treatment of psoriasis such as brodalumab33 (IL17 receptor antagonist); guselkumab,34 tildrakizumab35 and risankizumab36 (anti-IL23); and tofacitinib37 (Janus kinase 1 and 3 inhibitor). The biological agents currently available have already been shown to be effective in diseases other than psoriasis (sarcoidosis, lipoid necrobiosis, granuloma annulare, pityriasis rubra pilaris), and some of these may be approved in these indications in the near future.
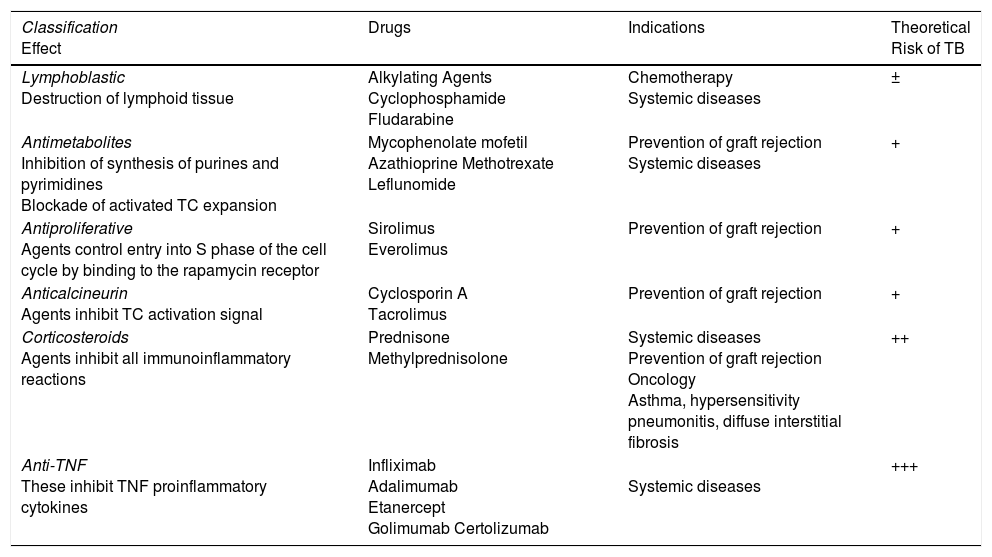
Tuberculosis and Biological TherapyCorticosteroid therapy in systemic diseases and immunosuppressant protocols to prevent graft rejection in transplantation have demonstrated in the past that TB is a real problem for patients treated with immunosuppressants38 (Table 3). The development of active TB in this population is a clear cause for concern: diagnosis may be delayed due to an atypical presentation,7,39 patients may present with adverse effects due to the underlying diseases or drug interactions, and the potential for TB transmission to the community should not be forgotten, including infection of other immunosuppressed patients who receive similar medical care in shared treatment areas.
Immunosuppressants That May Induce Tuberculosis.
| Classification Effect | Drugs | Indications | Theoretical Risk of TB |
|---|---|---|---|
| Lymphoblastic Destruction of lymphoid tissue | Alkylating Agents Cyclophosphamide Fludarabine | Chemotherapy Systemic diseases | ± |
| Antimetabolites Inhibition of synthesis of purines and pyrimidines Blockade of activated TC expansion | Mycophenolate mofetil Azathioprine Methotrexate Leflunomide | Prevention of graft rejection Systemic diseases | + |
| Antiproliferative Agents control entry into S phase of the cell cycle by binding to the rapamycin receptor | Sirolimus Everolimus | Prevention of graft rejection | + |
| Anticalcineurin Agents inhibit TC activation signal | Cyclosporin A Tacrolimus | Prevention of graft rejection | + |
| Corticosteroids Agents inhibit all immunoinflammatory reactions | Prednisone Methylprednisolone | Systemic diseases Prevention of graft rejection Oncology Asthma, hypersensitivity pneumonitis, diffuse interstitial fibrosis | ++ |
| Anti-TNF These inhibit TNF proinflammatory cytokines | Infliximab Adalimumab Etanercept Golimumab Certolizumab | Systemic diseases | +++ |
Abbreviations: TC, T-cell; TNF, tumor necrosis factor.
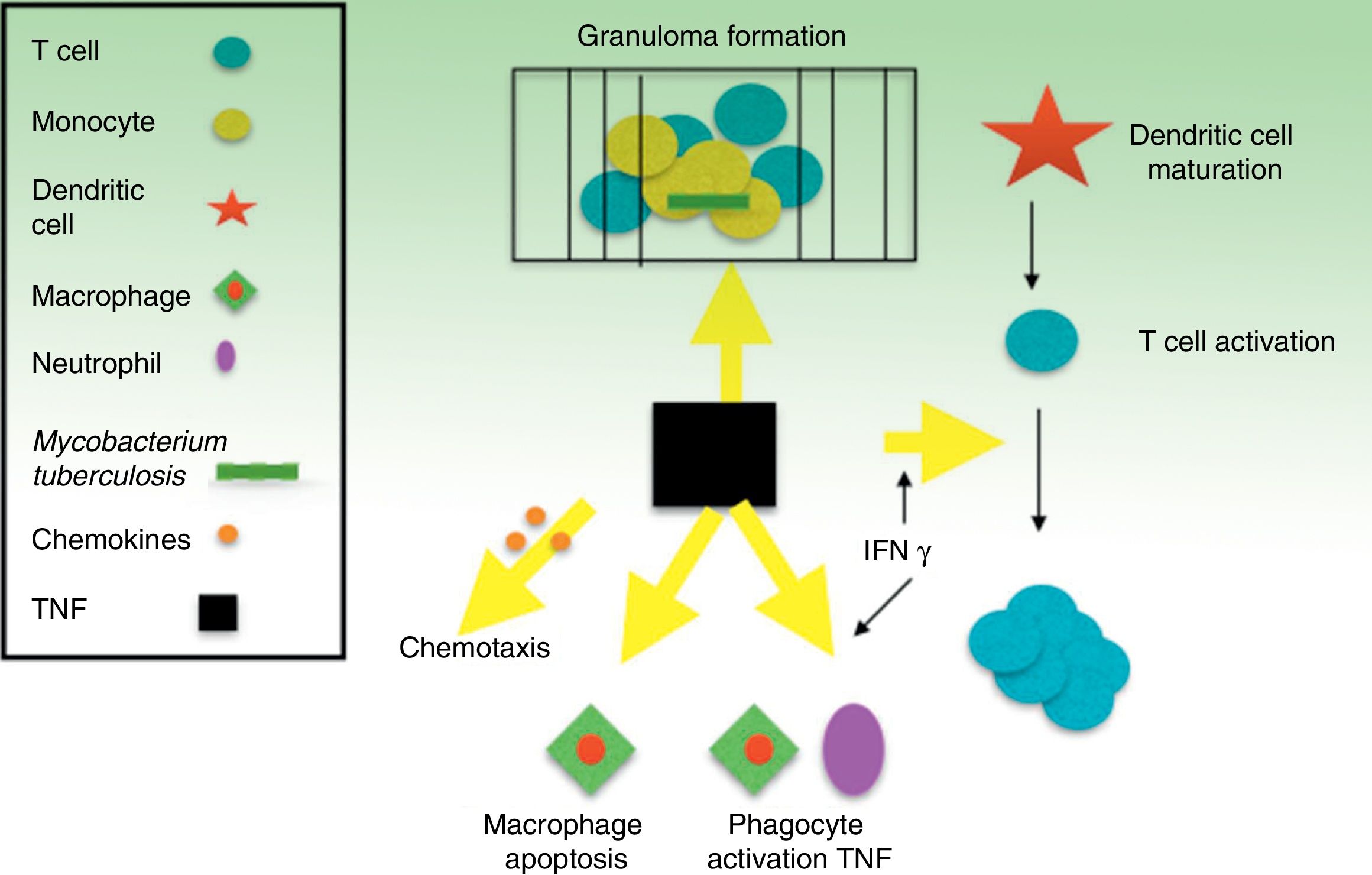
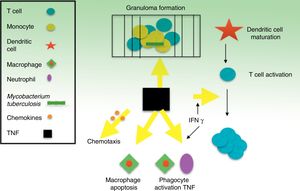
TNF is a potent modulator of early inflammatory response to a large variety of physical, environmental, infectious, and immunological stimuli. Specifically, TNF is a key part of the body's defense against M tuberculosis and, along with interferon (IFN) γ and other related cytokines, it plays an important role in the development and maintenance of granulomas as structures that compartmentalize and contain the tuberculosis bacillus during infection40,41 (Fig. 1). Inhibition of TNF and its regulatory network is the biological basis that appears to explain the 4- to 5-fold increase, on average, in TB incidence in patients with TB infection who receive infliximab.8 Cases of TB have also been reported with other lower affinity TNF inhibitors, such as etanercept, a fusion protein that binds to the soluble portion of the TNF receptor, and adalimumab, a monoclonal antibody like infliximab, but fully human.42 Only exceptionally, and without a clear causal relationship, has ustekinumab, a p40 protein inhibitor of IL12 and IL23, been related with possible reactivation of latent TB.43,44 IL17 inhibition, in contrast, does not appear to reactivate LTI according to in vitro models.45–47
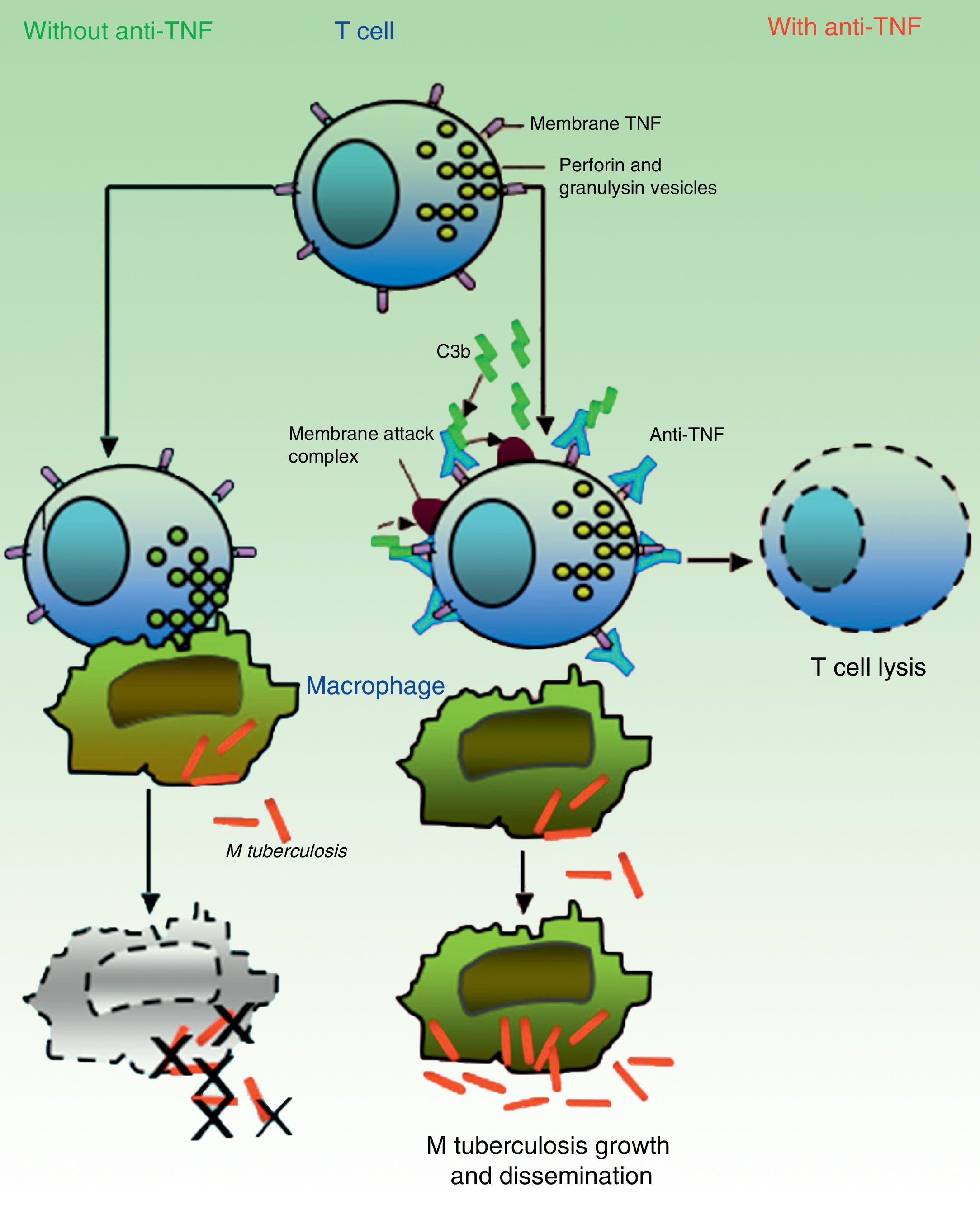
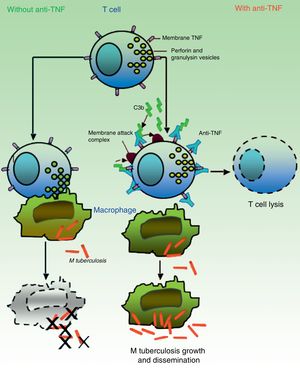
It is believed that granulomas are the structures that contribute to control of the intramacrophagic bacilli, as they provide a local environment in which antigen presenting cells (APCs) and lymphocytes interact to suppress growth and proliferation.7 A large part of the work for determining the TB reactivation mechanisms has focused on the role of TNF in the formation and maintenance of these structures. Bruns et al.,48 however, described an additional mechanism to explain the susceptibility to TB progression in individuals treated with infliximab. In cells from patients with a positive tuberculin test, they identified a subset of effector T cells, denominated CD8+ effector memory T cells (TEMRA), which are memory cells rich in granulysin, a protein with antimycobacterial properties, and they showed that TEMRA are selectively depleted by the effect of infliximab in patients with rheumatoid arthritis (Fig. 2).
In absence of anti-TNF, cytotoxic effector memory T cells (TEMRA) release granulomas with cytolytic enzymes (perforin, granulysin) that destroy macrophages infected by M tuberculosis as well as intra- and extracellular bacilli. In presence of anti-TNF, transmembrane TNF of TEMRA is bound to this antibody, favoring lymphocyte apoptosis. Depletion of cytotoxic T cells therefore prevents growth control and allows dissemination of M tuberculosis.
Source: Adapted from Miller and Ernst.41
Cell-based immunity is essential for control of TB infection and, for a long time now, it has been recognized that CD4+ T cells are important mediators of M tuberculosis immunity. More recently, the focus has been on specific CD8+ cells, but the meaning of this finding has yet to be determined, particularly in humans. It is believed that CD8+ cells directly limit growth of mycobacteria by causing death of infected cells,49,50 and also indirectly, through release of cytokines that promote activation of macrophages and chemokines that coordinate recruitment of other cells. Other studies indicate that CD8+ cells are probably less important during the acute phase of infection by M tuberculosis but essential during the chronic phase, and so they may help prevent TB reactivation.51,52 On assessment of the contribution of CD8+ cells to M tuberculosis immunity, it is important to remember that mice do not express granulysin, the cytolytic protein that, in humans, contributes to the death of M tuberculosis.49 Thus, studies in murine models may underestimate the importance of the antimycobacterial properties of CD8+ cells if their role is to be extrapolated to human immunity. Fewer studies have been performed in humans, but in vitro experiments show that TEMRA exhibit a high avidity for stains specific to granulysin and show higher levels both of cytotoxicity and antimycobacterial activity compared with other T cell subsets.53
The study by Bruns et al.48 goes beyond the definition of TEMRA as mere effectors with potential antimycobacterial importance, and attempts to explain the consequences of TNF blockade on these cells based on observations of M tuberculosis growth in cell culture. With these observations, an apparently new mechanism is described in which TNF is neutralized, thereby facilitating progression of latent infection to active infection. Previous studies in animal models had shown variable increases in bacillary load in absence of TNF.54 The results of these studies indicated that TNF contributes to direct control of intramacrophagic mycobacteria and indirect suppression of mycobacterial growth by modulating the formation and persistence of granulomas.
Risk of Tuberculosis With Anti-TNF AgentsTB infection is caused, in general, by inhalation of viable bacilli that usually persist in an inactive state known as LTI, although occasionally they can progress rapidly leading to active disease. Individuals with LTI remain asymptomatic and are not contagious. In most cases, initial infection by M tuberculosis can be contained by the hosts own defenses and the infection remains latent. However, this situation of latent infection can quickly turn into a disease state at any time.
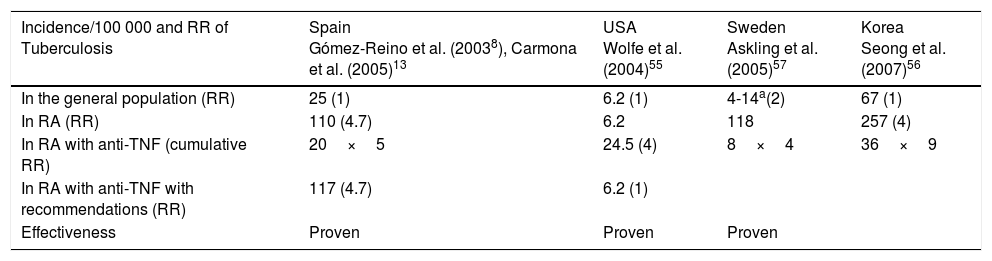
The risk of TB reactivation with use of anti-TNF agents depends on 2 variables: the immunomodulatory effect of treatment and the underlying prevalence of TB infection, or of individuals at risk of infection in a given population. Table 4 presents incidence data published in the first 10 years of use of anti-TNF agents for treatment of rheumatoid arthritis; note, however, that the results cannot necessarily be applied to dermatology patients.38,55–57
Incidence and Relative Risk of Tuberculosis in Rheumatoid Arthritis Before and After Introduction of Anti-TNF Agents: Impact of the Recommendations.
| Incidence/100 000 and RR of Tuberculosis | Spain Gómez-Reino et al. (20038), Carmona et al. (2005)13 | USA Wolfe et al. (2004)55 | Sweden Askling et al. (2005)57 | Korea Seong et al. (2007)56 |
|---|---|---|---|---|
| In the general population (RR) | 25 (1) | 6.2 (1) | 4-14a(2) | 67 (1) |
| In RA (RR) | 110 (4.7) | 6.2 | 118 | 257 (4) |
| In RA with anti-TNF (cumulative RR) | 20×5 | 24.5 (4) | 8×4 | 36×9 |
| In RA with anti-TNF with recommendations (RR) | 117 (4.7) | 6.2 (1) | ||
| Effectiveness | Proven | Proven | Proven |
Abbreviations: RA, rheumatoid arthritis; RR, relative risk; TNF, tumor necrosis factor.
Previous treatment for latent infection does not mean the patient is fully protected,58 and no standard period has been established for reactivation; rather, this may vary according to the drug used.59 The Italian multidisciplinary group for the detection of TB in patients receiving biological therapy (SAFEBIO) concluded in their guidelines that etanercept, among all anti-TNF agents, is the one with lowest risk of reactivation of LTI.12
Moreover, there is evidence that exposure to more than 1 anti-TNF drug may double the relative risk of exposure to a single drug for development of serious infections.60
Reinitiating Biological Treatment After Reactivation of TuberculosisIn the cohort study by Ozguler et al.,61 of 2754 patients treated with biological agents identified, 22 cases of active TB during treatment were identified. One of these patients with active disease died within a month due to miliary TB. In 16 of the 21 remaining patients (76%), after initial suspension of treatment, biological therapy was restarted: etanercept in 6 cases and rituximab in 5 cases were the most extensively used drugs. Of patients who restarted therapy, treatment was reinitiated while anti-TB therapy was ongoing in 4 patients, while 12 reinitiated after the course of anti-TB therapy. The median duration of follow-up after restarting treatment was 53 months (range 40-75 months). Only 1 of the patients who had restarted biological treatment presented with a second reactivation. This was satisfactorily treated with anti-TB therapy; subsequent follow-up of 33 months of biological maintenance therapy did not identify any further reactivation of infection. The authors concluded that restarting treatment with anti-TNF drugs, even during TB treatment, appears to be a viable option in patients who have previously developed TB infection during treatment with anti-TNF agents, although careful follow-up and monitoring are required.
The French RATIO group published the results of follow-up of 21 patients who had developed TB while receiving anti-TNF therapy.62 In 4 patients, it was necessary to restart biological therapy (in 3 of them during anti-TB treatment) and there was no recurrence of infection after a mean follow-up of 42.7 months. Aislandis et al.63 published a series of 5 patients with rheumatoid arthritis who had developed TB during anti-TNF therapy. In 3 of these, biological therapy had to be restarted, in all cases after completing anti-TB treatment, and there was no recurrence of infection after a mean follow-up of 24 months.
Therefore, it seems possible to restart biological therapy in cases of TB reactivation with an appropriate safety margin with respect to start of TB therapy.
Diagnostic Techniques for Latent Tuberculosis InfectionInvestigation of possible TB infection in candidates for biological treatment should start with evaluation of the potential risk of exposure to M tuberculosis. The highest-risk groups are summarized in Table 5.
Groups at High Risk of Conversion to Active Tuberculosis.
| Persons in recent contact with patients with TB disease |
| Persons born or who live in countries with a high prevalence of TB or those who travel frequently to such countries for business, family, or humanitarian reasons |
| Residents and workers in closed institutions such as prisons, hostels for the destitute, and social and health centers of any type |
| Abuse of alcohol and other harmful substances, while not forgetting that smokers develop TB more frequently than nonsmokers |
| Health workers, in particular those who attend patients with active TB |
| Persons with radiologic lesions suggestive of old TB, particularly if, as in the case of positive tuberculin, they have never received treatment |
| Very old and very young people, immunosuppressive diseases, and other comorbid conditions usually associated with increased risk of TB. This group includes patients with HIV infection, autoimmune diseases, and post-transplantation period, but also includes lung diseases (silicosis), chronic kidney disease, gastrectomy, diabetes, and certain tumors such as those of the head and neck |
Abbreviation: HIV, human immunodeficiency virus.
In absence of a gold standard for diagnosis of TB infection, current management of patients with autoimmune diseases who are candidates for anti-TNF treatment includes a directed medical history to investigate history of TB or latent infection, whether treated or not; assessment of the risk of exposure to possible active cases; the search for evidence of prior TB in the chest x-ray; and tests to detect LTI (tuberculin test [TT] or interferon-γ release assay [IGRA]).64
Tuberculin TestTT is a measure of delayed hypersensitivity response to intradermal inoculation with a purified protein derivative, a mixture of more than 200 proteins from M tuberculosis. Given that the antigens contained in the purified protein derivative are also present in other mycobacteria, bacillus Calmette and Guerin (BCG) vaccination may also lead to false positives. Moreover, TT sensitivity is compromised in patients receiving immunosuppressive treatment, with a high rate of false negatives.65 Studies in patients with rheumatoid arthritis have shown that the rate of TT false negatives can be as high as 40%.66 At times, repetition of the TT may be indicated to increase its sensitivity due to the booster effect on false negatives, but this also reduces its specificity, as it increases the number of false positives due to BCG vaccination and exposure to non-TB mycobacteria.67 Logistical drawbacks, including the need for a visit for the readout, and observer subjectivity on recording the result of the test may also impact its efficiency.
According to some authors, given that almost all candidates for anti-TNF treatment are receiving (or have received) another immunosuppressive agent, TT would not be the ideal test for LTI screening in patients with inflammatory diseases.7
The TT should never be repeated in patients with a positive result, as it will continue to be positive in most cases. There is a potential risk of severe reactions on repetition of the test while no clinical benefit is expected.
Tests Based on Interferon γ Release AssaySequencing of the M tuberculosis genome enabled better identification of the genes involved in its pathogenesis and awareness of the presence of regions of differing genetics. In the region known as RD1, proteins specific to M tuberculosis are secreted (ESAT-6 and CFP-10). These proteins act as specific antigens, inducing a Th1 type immune response with IFN-γ production in patients with M tuberculosis infection. This region, absent in all strains of Mycobacterium bovis-BCG and in almost all non-TB mycobacteria, except for Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium szulgai, is present in all known virulent strains of M tuberculosis.
IGRA techniques are therefore based on detection, in peripheral blood of infected individuals, of IFN-γ released by sensitized T cells in response to in vitro stimulation with M tuberculosis specific antigens. Two IGRA kits are currently available.
- -
QuantiFERON®-TB-Gold in tube (Cellestis Ltd., Carnegie, Australia)68 determines the total production of IFN-γ in infected individuals using an ELISA technique. A value of 0.35 IU/mL is considered positive.
- -
T-SPOT.TB® (Oxford Immunotec Ltd., UK)69 was developed by Lalvani at the end of the 1990s. The procedure for this kit is more laborious and requires prior separation of monocyte cells before incubation with ESAT-6 and CFP-10 antigens. The reading is done by the Elispot technique, in which each visible spot represents an IFN-γ secreting T cell. The result is considered positive if more than 6 spots are present.
Both techniques incorporate positive and negative controls able to detect false results due to anergy and immunological problems (reported as indeterminate results).70 In most guidelines, indeterminate results, which occur more frequently in patients with IMID, should be repeated and these are often confirmed as negative.71,72
The use of IGRA has a series of advantages over TT:
- 1.
Subjectivity in the interpretation of the results is avoided.
- 2.
The assay can be repeated if necessary.
- 3.
A reading visit at 48-72hours is not needed to obtain the result.
- 4.
Standardization and laboratory application are easy.
- 5.
Positive controls can be included when anergic patients are detected.
- 6.
The patient's privacy is respected.
Among the drawbacks, the most important is the cost, which is higher than for the TT. To reduce costs, depending on the capacity of the laboratory, assays can be delayed a reasonable time to enable batch analysis of as many samples as possible at the same time. In any case, implantation of this technique represents a diagnostic improvement and can spare health resources. Its greater specificity decreases the number of false positives compared with TT and, therefore, the costs derived from X-rays, visits, and unnecessary treatment for TB infection. On the other hand, it appears to be more sensitive than TT in those individuals with altered cell immunity at greatest risk of developing TB.73,74
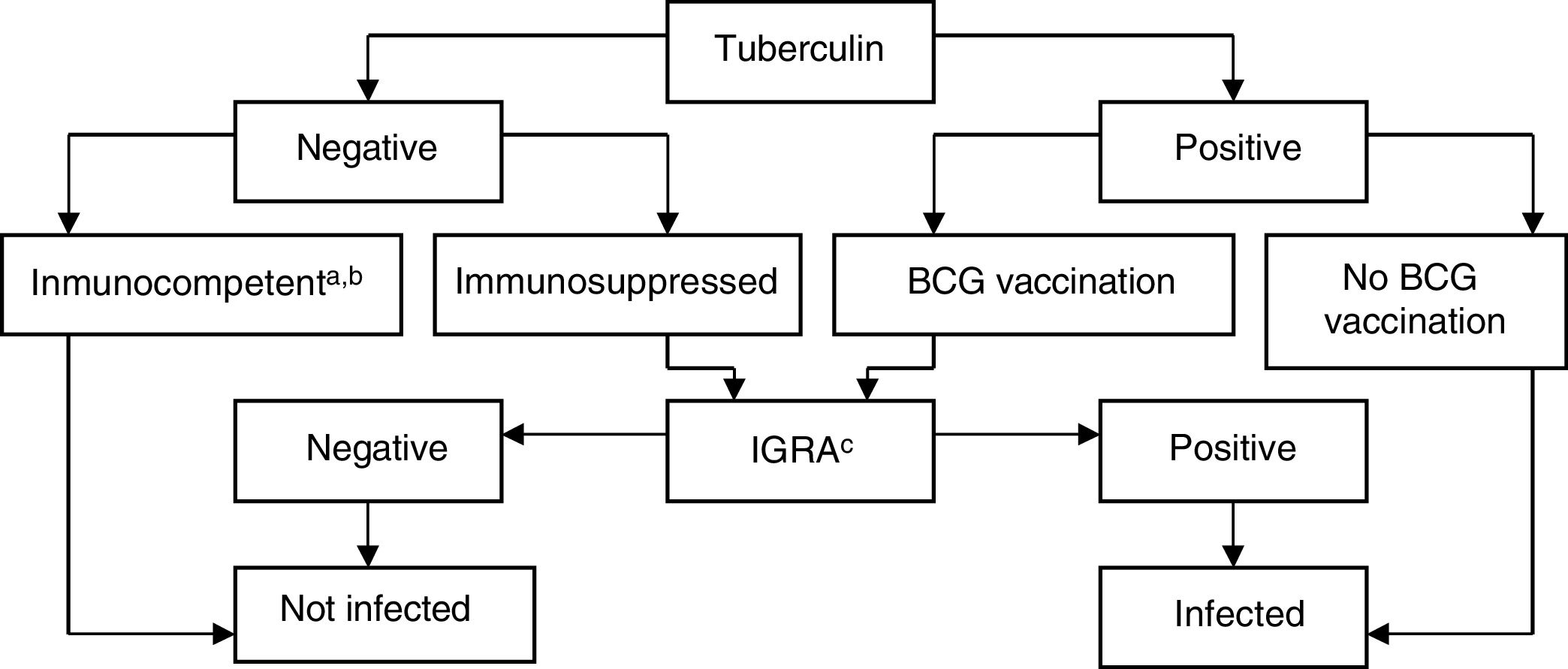
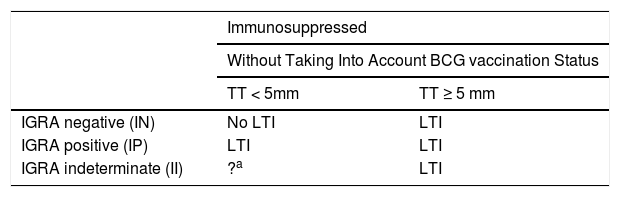
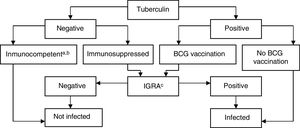
Interpretation of the Results in Candidates for Biological TreatmentsIGRAs are used as routine tests for diagnosis of latent infection in the United States of America, Canada, Australia, Japan, and some western European countries (United Kingdom, Italy, Germany, and Switzerland). Some countries use them instead of the TT, whereas others use them in combination. In Spain, it is recommended to complement the TT with IGRA to rule out false positives in candidates for chemoprophylaxis with prior BCG vaccination (to rule out false positives to tuberculin) and in those with a negative skin patch test and suspected immunosuppression (to rule out a false negative TT)75 (Fig. 3).
BCG, bacillus Calmette-Guerin; IGRA, interferon γ release assay.
a Patients with CD4+ counts ≥ 500 cells/mL are considered immunocompetent.
b In cases of severe immunosuppression (CD4+ count < 200 cells/mL), the probability of true infection should be assessed individually.
c IGRA measurement can be repeated periodically (depending on the individual risk in each patient) without any booster effect.
Source: Adapted from the Diagnostic and Treatment Guidelines for Tuberculosis of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR)..75
Although numerous articles have been published in recent years, data on the performance of these tests are limited and contradictory.74–80 The data from those studies enable the conclusion that: a) agreement between TT and IGRA is weaker in patients with systemic inflammatory diseases than in healthy subjects due to the lower prevalence of positive TT in the first group66,81–86; b) the extent of tuberculin response is significantly lower in patients than in healthy controls66; c) discordance between TT+ and IGRA- is observed in vaccinated patients; d) discordance between TT- and IGRA+ is often observed in patients receiving corticosteroid therapy86 (Table 6).
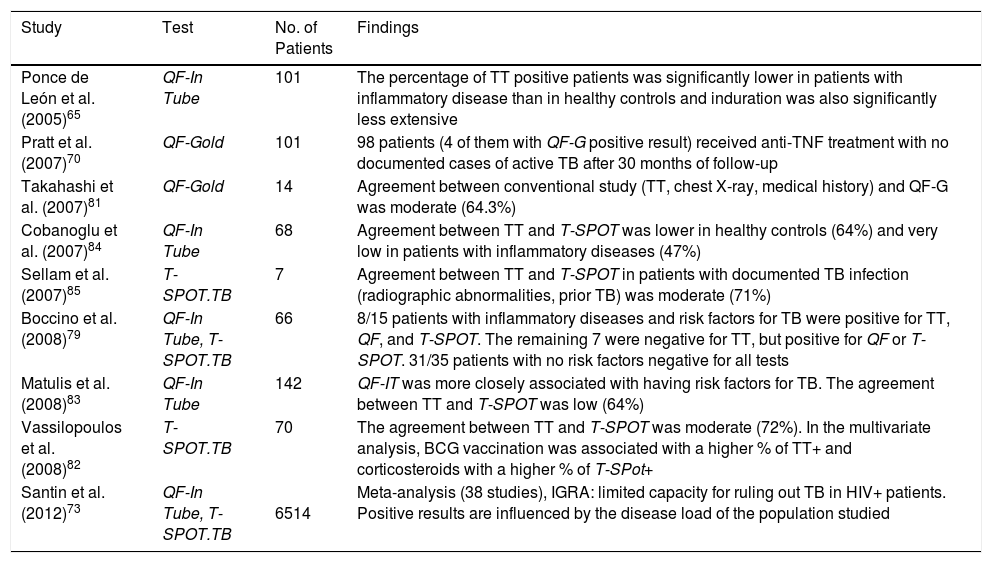
Results of Screening Tests for Latent Tuberculosis Infection in Patients with Systemic Inflammatory Diseases.
| Study | Test | No. of Patients | Findings |
|---|---|---|---|
| Ponce de León et al. (2005)65 | QF-In Tube | 101 | The percentage of TT positive patients was significantly lower in patients with inflammatory disease than in healthy controls and induration was also significantly less extensive |
| Pratt et al. (2007)70 | QF-Gold | 101 | 98 patients (4 of them with QF-G positive result) received anti-TNF treatment with no documented cases of active TB after 30 months of follow-up |
| Takahashi et al. (2007)81 | QF-Gold | 14 | Agreement between conventional study (TT, chest X-ray, medical history) and QF-G was moderate (64.3%) |
| Cobanoglu et al. (2007)84 | QF-In Tube | 68 | Agreement between TT and T-SPOT was lower in healthy controls (64%) and very low in patients with inflammatory diseases (47%) |
| Sellam et al. (2007)85 | T-SPOT.TB | 7 | Agreement between TT and T-SPOT in patients with documented TB infection (radiographic abnormalities, prior TB) was moderate (71%) |
| Boccino et al. (2008)79 | QF-In Tube, T-SPOT.TB | 66 | 8/15 patients with inflammatory diseases and risk factors for TB were positive for TT, QF, and T-SPOT. The remaining 7 were negative for TT, but positive for QF or T-SPOT. 31/35 patients with no risk factors negative for all tests |
| Matulis et al. (2008)83 | QF-In Tube | 142 | QF-IT was more closely associated with having risk factors for TB. The agreement between TT and T-SPOT was low (64%) |
| Vassilopoulos et al. (2008)82 | T-SPOT.TB | 70 | The agreement between TT and T-SPOT was moderate (72%). In the multivariate analysis, BCG vaccination was associated with a higher % of TT+ and corticosteroids with a higher % of T-SPot+ |
| Santin et al. (2012)73 | QF-In Tube, T-SPOT.TB | 6514 | Meta-analysis (38 studies), IGRA: limited capacity for ruling out TB in HIV+ patients. Positive results are influenced by the disease load of the population studied |
Abbreviations: Anti-TNF, anti-tumor necrosis factor; BCG, bacillus Calmette-Guerin; HIV, human immunodeficiency virus; IGRA, interferon γ release assay; QF, Quantiferon; TB, tuberculosis; TT, tuberculin test.
Due to the vulnerability of these patients to TB when they are receiving anti-TNF treatment, it seems prudent to perform the 2 tests (TT and IGRA) in parallel to maximize diagnostic sensitivity of screening, at least, until evidence of the utility of IGRA in this population has been consolidated. It is important to mention that when the 2 tests are performed sequentially, IGRAs should be performed first due to the booster effect of the TT.87,88 It is not known how long the booster effect persists and whether it depends on the quantity of purified protein derivative administered or its characteristics.
Given that some patients with latent infection may have false negatives for IGRAs and true positives for TT, and vice versa, the dual screening strategy would enable clinicians to offer preventive treatment to all patients with latent infection (Table 7).
Interpretation of the Results of Screening in Immunosuppressed Patients.
| Immunosuppressed | ||
|---|---|---|
| Without Taking Into Account BCG vaccination Status | ||
| TT < 5mm | TT ≥ 5 mm | |
| IGRA negative (IN) | No LTI | LTI |
| IGRA positive (IP) | LTI | LTI |
| IGRA indeterminate (II) | ?a | LTI |
Immunosuppressed patients with 1 or 2 positive screening tests should receive treatment for LTI with any of the recommended regimens. Doubtful or indeterminate results should be repeated (complement TT with IGRA or T-SPOT.TB).
Abbreviations: IGRA interferon γ release assay; LTI latent tuberculosis infection; TT tuberculin test.
The results of the IGRAs in patients who are in treatment with anti-TNF agents remain difficult to interpret. One proposed strategy was to repeat the tests after starting biological therapy to detect false negatives. The available data from recent studies89–91 show conversions and reversions in both tests related to reproducibility problems and changes in the patients¿ immune status. Given this variability, it is not recommended to repeat the test for follow-up of patients during treatment.
Treatment of Latent Tuberculosis InfectionThe identification and treatment of LTI greatly reduces the risk of reactivation and, thus, has the potential to protect the health of infected individuals as well as preventing further infection by reducing the number of possible sources of contagion.92,93
After treatment of LTI, the durability of protection against reactivation is variable and depends on the prevalence of TB in the area and the risk of re-exposure. It is assumed that treatment for LTI can confer lifelong protection against disease. Among Eskimos in Alaska, for example, the protective effect of isoniazid (INH) has been shown to last more than 20 years.94,95
The treatment of latent infection is only started once active TB has been excluded.
Regimens for LTI treatment include INH for 9 months, rifampicin (RIF) monotherapy for 4 months, or INH with RIF for 3 months.96
Isoniazid for 9 MonthsThe INH dose used is 5mg/kg/d with a maximum of 300mg/d in adults. In children under 16 years of age, the dose used should be 10mg/kg/d. The efficacy of INH for reducing the incidence of active TB (compared with placebo) is between 60% and 90%. However, given that not all patients complete the treatment course, the efficacy of treatment is only approximately 60% on average.94
The only study that compares the efficacy of different durations of INH treatment showed efficacy was 65% for the 6 month regimen and 75% for the 12 month regimen (although the difference was not statistically significant). By extrapolation of the data from randomized trials, the optimum duration of INH treatment for LTI was considered to be 9 months, as the study showed similar efficacy values from 9 months onwards.94,97
The most important side effect of INH is hepatitis, with an incidence of 8%. Most cases are asymptomatic and associated with slight liver enzyme elevations that do not usually exceed 10 times the upper limit of normal.98,99 The most important risk factor for development of INH-induced hepatitis is alcohol consumption. Patients should be advised to abstain from alcohol consumption while they are taking INH. Peripheral neuropathy presents in up to 2% of patients who take INH.100 This is caused by interference in pyridoxine metabolism and can be prevented by pyridoxine supplements (25 and 50mg/d). This is particularly important for patients with conditions that may predispose them to neuropathy (including diabetes, uremia, alcohol abuse, malnutrition, human immunodeficiency virus infection) as well as pregnant patients with a prior history of convulsions. Pyridoxine should also be administered to babies of breast-feeding mothers who receive INH. Spain has INH formulations that already include vitamin B6, and so this problem is minimized.
The preferred treatment regimen for most individuals with LTI is INH daily for 9 months, as recommended by the Centers for Disease Control (CDC).92,101 A 6-month course of INH also provides substantial protection. In the context of adherence concerns, clinicians may opt to concentrate their efforts in guaranteeing 6 months of therapy. However, regimens shorter than 9 months are not recommended for children or for patients with fibrotic lesions in chest X-ray or in immunosuppressed patients. For patients with poor adherence to treatment, directly observed therapy may be the preferred approach, with administration once or twice a week.
Rifampicin for 4 MonthsThe usual dose is 10mg/kg/d with a maximum of 600mg/d. The efficacy of RIF for reducing the incidence of active TB is estimated to be similar to INH.102,103 Although data are limited, RIF appears to be well-tolerated, with a low rate of hepatotoxicity.102 The drawbacks to routine adoption of RIF for the treatment of latent TB infection include the possibility of underdiagnosing cases of active TB that could then relapse with strains resistant to RIF. Although this could also occur in cases treated with INH, surprisingly, the incidence of relapse with INH has been very low.104 Interactions with other drugs and its elevated price have also been factors for concern in the case of RIF. Thus, there are important and potentially prolonged interactions with other drugs, including warfarin, oral contraceptives, certain antihypertensive agents, antiarrhythmic agents, antidepressants and anticonvulsants, methadone, and the protease inhibitor class of antiretrovirals.105,106
Isoniazid and Rifampicin for 3 MonthsData on the use of INH and RIF together are limited, but some studies have shown that the combination is an effective regimen with good adherence and well tolerated.107 A meta-analysis of small studies showed that it is just as effective and no more toxic than the 9-month regimen with INH.108 In a prospective, randomized study in individuals infected by the human immunodeficiency virus, a regimen of 3 months of INH and RIF daily offered 60% protection.109
Finally, the combination of RIF with pyrazinamide should not be used for the treatment of ILT, due to the possibility of severe hepatotoxicity.96
Treatment of Latent Tuberculosis Infection in Multidrug Resistant StrainsFor exposed patients who supposedly are infected with mycobacteria resistant to multiple agents, treatment of LTI should be individualized, based on the susceptibility profile of the index case.110,111 Two drugs should always be chosen to which the organism is susceptible, for example, a combination of pyrazinamide and ethambutol or a fluoroquinolone with antituberculous activity (such as levofloxacin).112 The duration of treatment has not been defined, although from 6 to 12 months seems to be the most reasonable. Patients with LTI due to multidrug resistant strains should receive carefully monitored treatment and be followed closely due to the risk of liver toxicity. In the case of extreme multidrug-resistant strains, with resistance to several drugs, prophylaxis in contacts may be very difficult and no regimens have been tested beyond the first-line drugs.
Monitoring of Treatment for Latent Tuberculosis InfectionPatients in treatment for LTI should be supervised monthly with clinical examination and liver testing. Patients should also be educated about the symptoms of hepatitis and instructed to suspend treatment and seek medical attention quickly to reduce the risk of progression to serious disease.113
Treatment of LTI should be discontinued in symptomatic patients with serum transaminase levels in excess of 3 times the upper limit of normal or in asymptomatic patients with serum transaminase levels more than 5 times the upper limit of normal. Treatment should not be restarted under any circumstances.
A common dilemma is whether to continue LTI treatment after having started complete treatment for suspicion of active TB which is finally not confirmed (negative cultures or histology). The duration of combination therapy in the initial phase administered may apply to the total duration of treatment for LTI. Subsequent options include interrupting treatment if RIF and pyrazinamide have been given for less than 2 months, continuing treatment with RIF for a total of 4 months (with or without INH), or continuing with INH treatment for a total of 9 months.114
In the case of regimens with INH (9 or 6 months), treatment can be restarted where it was left off if less than 3 months have been lost. Treatment for LTI should be reinitiated from the start if more than 3 months have elapsed. For treatment of LTI with regimens of 3 or 4 months, treatment can be restarted from where it left off if less than 2 months of the course remain.
Treatment of Latent Tuberculosis Infection in Candidates for Biological TherapiesThe CDC recommends treatment of LTI of all patients who are planning to take a TNF inhibitor and who have a positive TT result (induration ≥5mm) or a positive IGRA result115,116 and in the case of patients with negative TT or IGRA but with evidence of previous TB disease in the chest X-ray (for example, regional fibrosis with or without enlarged hilar lymph nodes) or if there is epidemiological evidence of prior exposure to TB (for example, after having been in close contact with a TB case).
Patients who are candidates to receive anti-TNF agents with an indication for LTI treatment in general should receive standard therapy, that is, INH for 9 months. The recommendation about what the duration of therapy for LTI should be before starting anti-TNF has not been established, but most authors propose that patients should receive at least 1 month of treatment for LTI before starting anti-TNF therapy, whenever this is possible.14,117
Treatment with LTI will not protect against TB in the case of recently acquired infection except, perhaps, during the time that the treatment is administered. Therefore, patients who take anti-TNF agents should avoid exposure to TB (for example, occupational exposure such as in health centers with a high prevalence of TB, homeless shelters, prisons, travel to regions with a high prevalence of TB). Treatment with INH of latent infection will likewise not protect against reactivation of the infection by INH-resistant strains.118 There are no studies to enable recommendation of combined regimens of LTI treatment in these patients, although the RIF plus INH regimen has been used by expert groups in a large number of patients, without having detected cases of active TB after starting treatment.
Recommendations of the Consensus DocumentTable 8 summarizes the recommendations of the panel of experts from the scientific societies participating in this multidisciplinary consensus document. The aim is to guide dermatology specialists in the assessment of risk of developing TB and to prevent disease through the diagnosis and treatment of LTI.
Final Recommendations with Degree of Evidence.
| All candidates for biological therapies should be studied for diagnosis of LTI as these patients belong to one of the highest risk groups for developing TB.8,9 | AIII | |
| The risk of these patients falling ill appears to be depend on the anti-TNF agent used: infliximab and adalimumab are those with highest risk detected8,42 | AIII | |
| The diagnostic methods for LTI detection are based on75: | Active search for TB history in the patient's medical record as well as contacts with active TB | AIII |
| Chest X-ray can detect possible old TB lesions. In the case of doubt, it is advised to complete the study with chest CT, given that this technique is superior to chest X-ray for detection of early radiologic signs of active TB or old lesions | ||
| Simultaneous performance of IGRA and TT. Any positive result in one of these tests is considered indicative of LTI | ||
| Patients diagnosed with IMIDs have a higher rate of false negatives with TT and IGRA.71,72 | AIII | |
| There is no evidence that repeating TT (booster effect) increases the sensitivity of the test in patients with IMID but that it does reduce specificity, and so currently, with the use of IGRA, the TT is not recommended67 | CIII | |
| IGRA should be performed before TT, due to the booster effect detected for IGRAs.87,88 | AIII | |
| The specificity and sensitivity of the 2 IGRA techniques for the diagnosis of LTI are similar in patients with IMID; as the sensitivity of T.SPOT.TB is somewhat higher in patients treated with corticosteroids, use of this test should be considered in these patients73,74 | BIII | |
| Indeterminate results should always be confirmed with a second measurement that, in most cases, is negative71,72 | AIII | |
| Negativity in the TT and IGRA does not rule out LTI.66,66,70,82 | AIII | |
| Preventive treatment is recommended in all candidates for therapy who presented with suspicion of LTI, after excluding active TB, in any of the diagnostic tests92,93,96,115,116 | AIII | |
| Monitoring of treatment should be done monthly. In the case of INH liver toxicity, a 4-month RIF regimen is recommended113 | AIII | |
| A period of 4 weeks of treatment for LTI is considered safe (by most experts) for starting treatment with anti-TNF61 | AIII | |
| The study and screening for LTI, after onset of anti-TNF treatment and during maintenance, as a diagnostic strategy in initial false negatives is not supported by evidence currently available. It is recommended to repeat the screening study if there are changes in clinical symptoms or potential exposure to Mycobacterium tuberculosis due to travel to regions where infection is endemic88–91 | AIII | |
| If the patient is diagnosed with active tuberculosis disease, anti-TNF treatment should be suspended; it is recommended to delay reinitiating until the entire TB treatment cycle has been completed. There is no evidence that the duration of treatment for TB disease should be modified in this context61 | AIII | |
| Patients who have correctly completed treatment for TB do not appear to be at a higher risk of relapse when anti-TNF treatment is initiated94,95 | AIII | |
Abbreviations: CT, computed tomography; IGRA, interferon γ release assay; IMID, immune-mediated inflammatory disease; INH, isoniazid; LTI, latent tuberculosis infection; TNF, tumor necrosis factor, RIF, rifampicin; TB, tuberculosis; TT, tuberculin test.
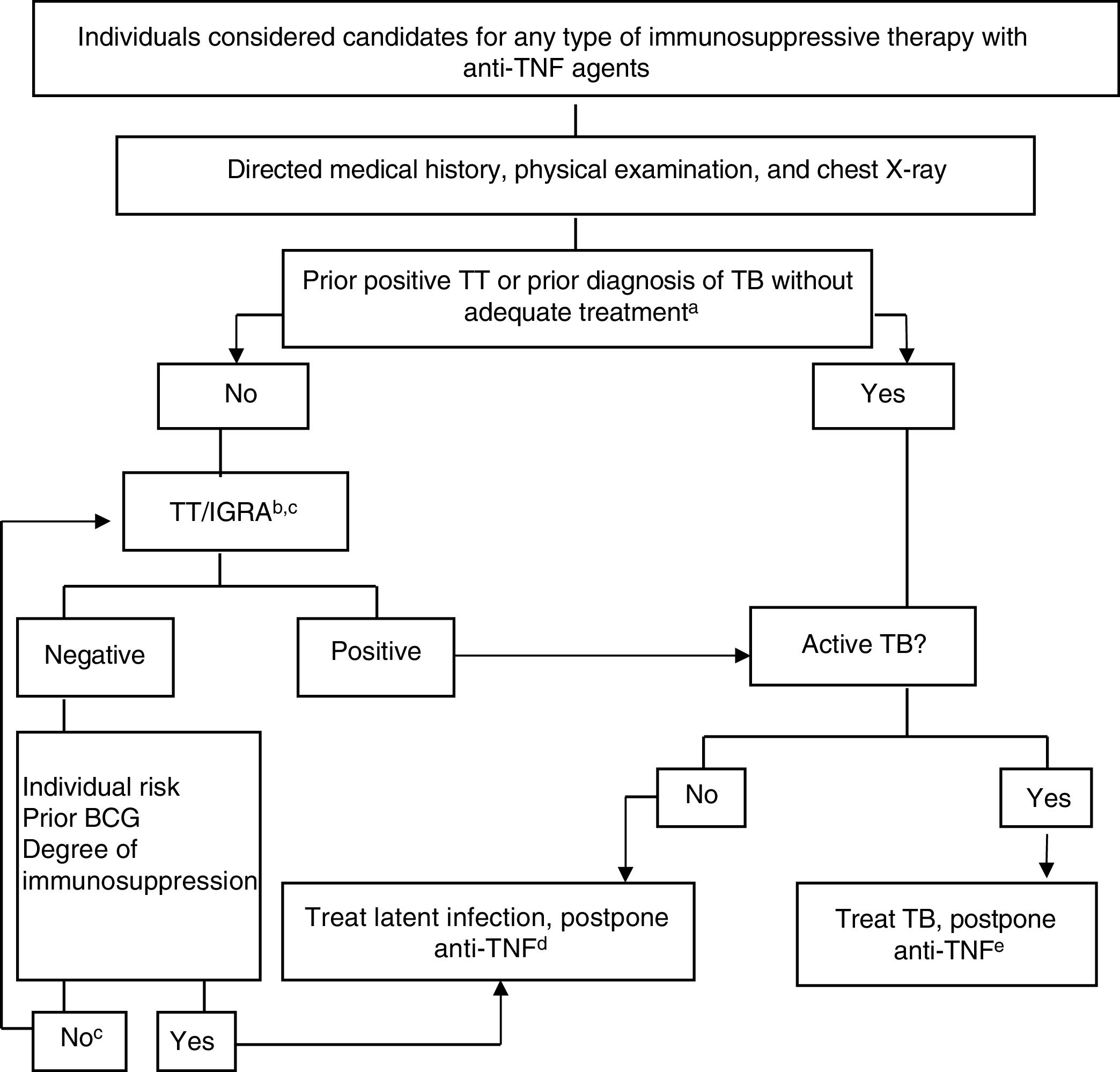
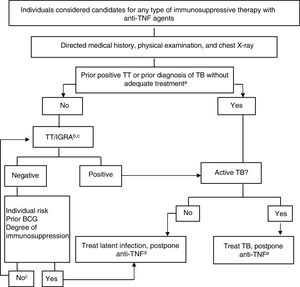
Figure 4 shows the algorithm proposed for assessment of TB infection in candidates for biological therapy.119,120
Provisional algorithm for assessment of TB infection in candidates for anti-TNF treatment.
a Appropriate treatment for TB is defined as ≥6 months of treatment with first-line drugs, including ≥2 months of the combination of rifampicin, isoniazid, pyrazinamide and ethambutol. Appropriate treatment for latent infection can be administration of ≥6 months of isoniazid, 3 months of isoniazid and rifampicin, or 4 months of rifampicin alone.
b The risk of latent infection is determined by weighting factors such as exposure to known contagious case, age, country of origin, and work and social history of the individual, including travel to endemic countries and repeated exposure to collectives at risk (closed institutions, homeless, drug users).
c In those infected many years earlier, TT can be negative and become positive in a second TT (booster phenomenon). With the availability of IGRAs, it would seem more practical to use these as complementary tests rather than performing a second TT, both in patients aged more than 60 years and in immunosuppressed patients due to different causes, regardless of age.
d There are no conclusive data to establish a safe period between the start of treatment for latent infection and the start of anti-TNF therapy. Four weeks delay is considered usual practice and safe by most experts.
eActive TB treatment should be completed before starting biological therapy. See text for interpretation and extension of this algorithm.
Anti-TNF, anti-tumor necrosis factor; BCG, bacillus Calmette-Guerin; IGRA, interferon γ release assay; TB, tuberculosis; TT, tuberculin test.
Source: Adapted from Winthrop..120
Esteban Daudén declares he is a member of advisory boards, consultant, grant recipient, research support recipient, participant as an investigator in clinical trials, and recipient of fees for talks with the following pharmaceutical companies: Abbvie (Abbott), Amgen, Janssen-Cilag, Leo Pharma, MSD, Pfizer, Novartis, Celgene, and Lilly.
Carlos Taxonara Samso declares he is a consultant and speaker for MSD, AbbVie, Jannsen, Takeda, and Pfizer.
Francisco Javier López Longo declares having been an occasional speaker for Abbvie, Actelion, Bristol-Myers-Squibb, GSK, MSD, Pfizer, Roche Farma, and UCB and having received research funding from Abbvie and GSK.
The remaining authors declare that they have no conflicts of interest.
Please cite this article as: Rodríguez-Jiménez P, Mir-Viladrich I, Chicharro P, Solano-López G, López-Longo FJ, Taxonera C, et al. Consenso multidisciplinar sobre prevención y tratamiento de la tuberculosis en pacientes candidatos a tratamiento biológico. Adaptación al paciente dermatológico. Actas Dermosifiliogr. 2018;109:584–601.