In recent years, the use of platelet-rich plasma has increased notably in a range of diseases and settings. Uses of these products now go beyond skin rejuvenation therapy in patients with facial ageing. Good outcomes for other dermatological indications such as skin ulcers and, more recently, alopecia have been reported in case series and controlled studies. However, these indications are not currently included in the labeling given that stronger scientific evidence is required to support their real benefits. With the increased use of these products, dermatologists need to become familiar with the underlying biological principles and able to critically assess the quality and outcomes of the studies of these products in different skin diseases.
La aplicación del plasma rico en plaquetas ha experimentado un notable auge en los últimos años en una amplia variedad de enfermedades y situaciones clínicas. Su empleo en dermatología va más allá de su asociación con el envejecimiento facial. En la literatura se pueden encontrar series de casos y estudios controlados que muestran buenos resultados en aplicaciones diversas, como las úlceras cutáneas y, más recientemente, la alopecia. Sin embargo, estas indicaciones no están reconocidas en la ficha técnica en el momento actual, a falta de poder demostrar sus beneficios reales con mayor evidencia científica. Ante la expansión en el uso de esta técnica resulta fundamental el conocimiento de sus fundamentos biológicos y la evaluación de la calidad y de los resultados de los trabajos que estudian su aplicación en diferentes enfermedades cutáneas.
Although mention of platelet-rich plasma (PRP) may initially bring to mind supposedly novel applications in esthetic medicine, and facial aging in particular, the product has actually been used for many years in wide range of indications. After the discovery in the 1980s of the release of bioactive molecules with action in damaged tissue such as skin ulcers, PRP started to be used in regenerative medicine.1 Ten years later, PRP started to be used in maxillofacial surgery, taking advantage of the potential of fibrin for adherence and its hemostatic properties.2 Clinical observation revealed PRP could stimulate cell proliferation and have anti-inflammatory characteristics.3
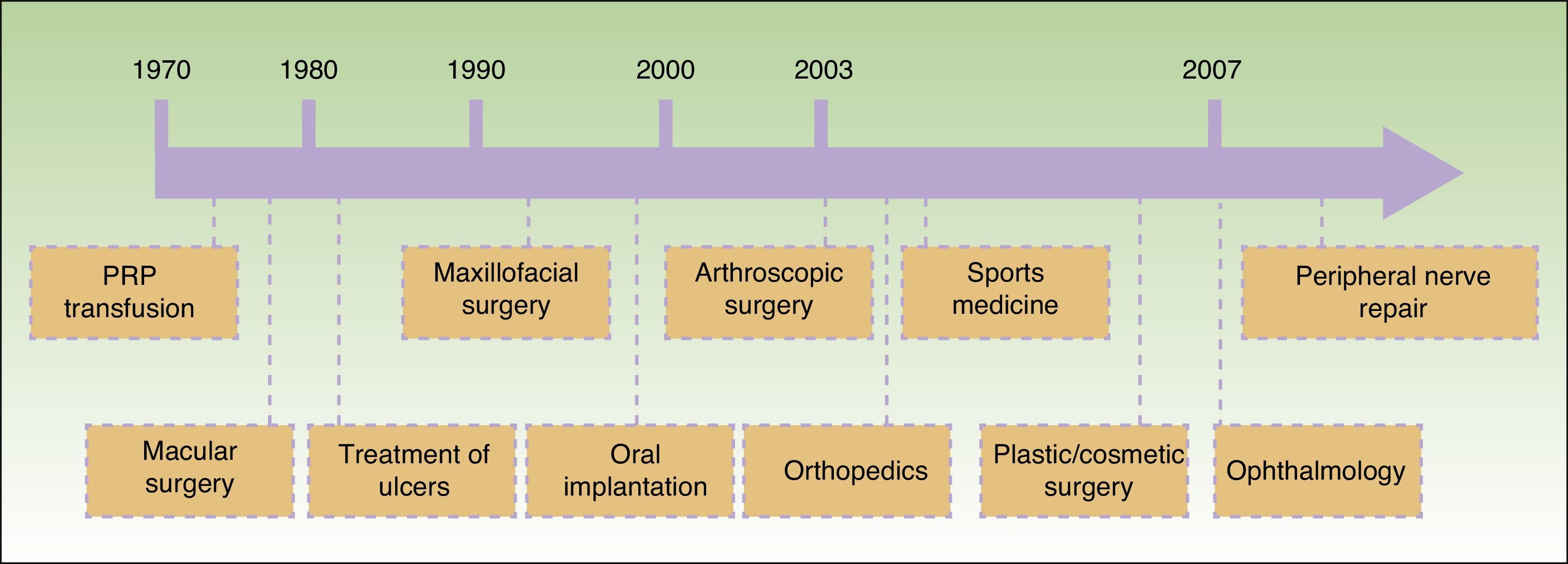
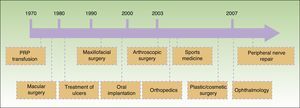
Since Anitua2 reported an outpatient method for obtaining PRP for application in implantology in 1999, different techniques have been developed and the range of applications has been extended. A timeline for the introduction of these different applications is shown in Figure 1. At least 16 different systems for obtaining PRP are available commercially at present.
Temporal sequence of application of platelet-rich plasma (PRP) in different fields of medicine.3
This apparent widespread availability is at odds with the limited scientific evidence to support the different suggested applications. Most of the studies in the literature reflect benefits, at times rather striking, of application of PRP. However, few of these clinical trials have a robust design that enables the size of the effect to be evaluated. Furthermore, there is little consensus on the large variety of methodologies available. Thus, there is a lack of standardization in the use of PRP and, therefore, generation of readily reproducible scientific evidence is hindered. In this context, the Spanish Agency for Medicines and Health Products (AEMPS) issued a report in May 2013 with the aim of establishing a framework for the use of PRP in Spain, the obligations of the manufacturers, and the information that the patients treated with PRP should receive. This document recognizes PRP as a drug product for human use.4
The objective of this review is, first, to explain the mechanism of action of PRP in tissue regeneration and, second, to summarize the scientific evidence available at present for the different proposed indications.
Platelets in Tissue RegenerationIn addition to their recognized role in hemostasis, platelets have other essential functions in tissue regeneration. After tissue and vascular damage, platelets become activated and aggregate as part of their hemostatic function. This leads to secretion of proteins and other biologically active molecules which, in turn, trigger cascades of secondary messengers implicated in the tissue healing process. The theoretical basis for the biological benefit of PRP is that concentrations above the physiological one of platelets and plasma proteins may accelerate the repair process. In addition, reinforcement of the fibrin mesh may enable the viability of sustained release of bioactive molecules to be maintained.5–8
Definition of Platelet-Rich PlasmaThere is no consensus on the definition of PRP. Some investigators have suggested that PRP should refer to the fraction with a platelet concentration 3 to 5 times greater than normal levels. However, the most accepted definition at present characterizes PRP as a volume of autologous plasma that contains a platelet concentration above basal concentration (150 000-350 000/μL).8
The platelet, leukocyte, and growth factor concentrations vary according to the concentration of platelets used in the preparation. As a result, the nomenclature for PRP products makes reference to the different fractions that can be obtained according to the method used: plasma-rich growth factors (PRGF), platelet-rich plasma and growth factors (PRPGF), platelet-rich plasma (PRP), platelet-poor plasma (PPP), leukocyte-rich platelet-rich plasma (LR-PRP), and leukocyte-poor platelet-rich plasma (LP-PRP).
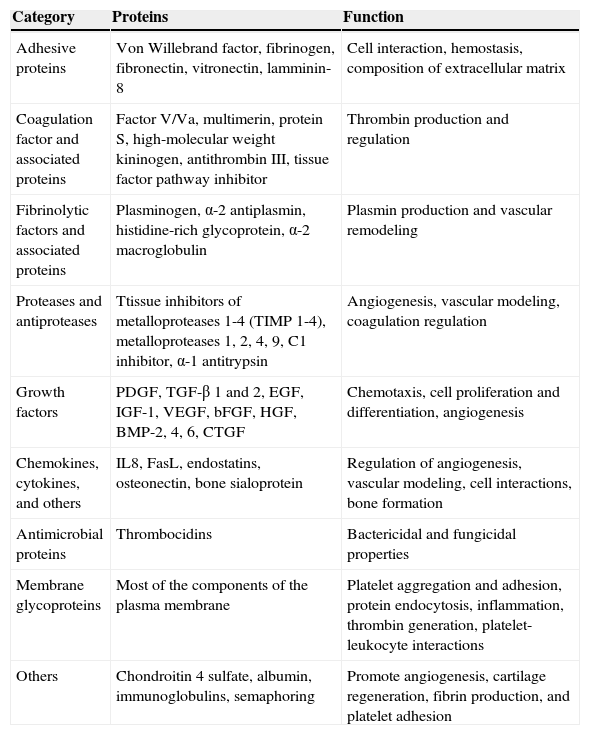
Bioactive Molecules in Platelet-Rich PlasmaIn addition to the known growth factors, PRP contains other bioactive molecules with an important role in tissue healing. These include platelet-derived growth factor (PDGF), transforming growth factor (TGF), platelet factor 4 (PF4), interleukin (IL) 1, platelet-derived angiogenesis factor (PDAF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), platelet-derived endothelial growth factor (PDEGF), epithelial cell growth factor (ECGF), and insulin-like growth factor (IGF). These molecules, and other bioactive molecules, have different important functions in the local regeneration environment such as proliferation, migration, and cell differentiation and angiogenesis. It is difficult to define the specific functions of each factor as these overlap to a certain extent (Table 1).
Classification and Functions of Bioactive Molecules Present in Platelet-Rich Plasma.
| Category | Proteins | Function |
|---|---|---|
| Adhesive proteins | Von Willebrand factor, fibrinogen, fibronectin, vitronectin, lamminin-8 | Cell interaction, hemostasis, composition of extracellular matrix |
| Coagulation factor and associated proteins | Factor V/Va, multimerin, protein S, high-molecular weight kininogen, antithrombin III, tissue factor pathway inhibitor | Thrombin production and regulation |
| Fibrinolytic factors and associated proteins | Plasminogen, α-2 antiplasmin, histidine-rich glycoprotein, α-2 macroglobulin | Plasmin production and vascular remodeling |
| Proteases and antiproteases | Ttissue inhibitors of metalloproteases 1-4 (TIMP 1-4), metalloproteases 1, 2, 4, 9, C1 inhibitor, α-1 antitrypsin | Angiogenesis, vascular modeling, coagulation regulation |
| Growth factors | PDGF, TGF-β 1 and 2, EGF, IGF-1, VEGF, bFGF, HGF, BMP-2, 4, 6, CTGF | Chemotaxis, cell proliferation and differentiation, angiogenesis |
| Chemokines, cytokines, and others | IL8, FasL, endostatins, osteonectin, bone sialoprotein | Regulation of angiogenesis, vascular modeling, cell interactions, bone formation |
| Antimicrobial proteins | Thrombocidins | Bactericidal and fungicidal properties |
| Membrane glycoproteins | Most of the components of the plasma membrane | Platelet aggregation and adhesion, protein endocytosis, inflammation, thrombin generation, platelet-leukocyte interactions |
| Others | Chondroitin 4 sulfate, albumin, immunoglobulins, semaphoring | Promote angiogenesis, cartilage regeneration, fibrin production, and platelet adhesion |
Source: Anitua et al.7
These preparations have also been attributed antibacterial properties, which have been associated with both certain platelet proteins and with leukocyte action of white blood cells in the PRP concentrates.
The proteins that act to promote cell adhesion (fibrin, fibronectin, and vitronectin) are another essential component of PRP. These provide the structural support necessary for cell migration and for proliferation and 3-dimensional growth of tissues over these structures. Therefore, PRP has effects not only directly related to the target cells for the various growth factors but also has a broader effect as an extracellular matrix for the stimulation and repair and/or regeneration of the tissue.5–8
Procedure for Obtaining Platelet-Rich PlasmaTo obtain PRP, the following steps, which may vary according to the technique are used. The method can prepare the product from scratch or follow the manufacturer instructions when disposable kits are used. First, blood is taken from a vein of a patient and collected in sterile tubes with citrate as anticoagulant. The tubes are then centrifuged in a conventional centrifuge. The duration, speeds, and number of centrifuge steps depend on the method used. To avoid fragmentation of the platelets and the subsequent early release of the secreted proteins, with corresponding negative impact on their bioactivity, low centrifugation speeds are recommended.
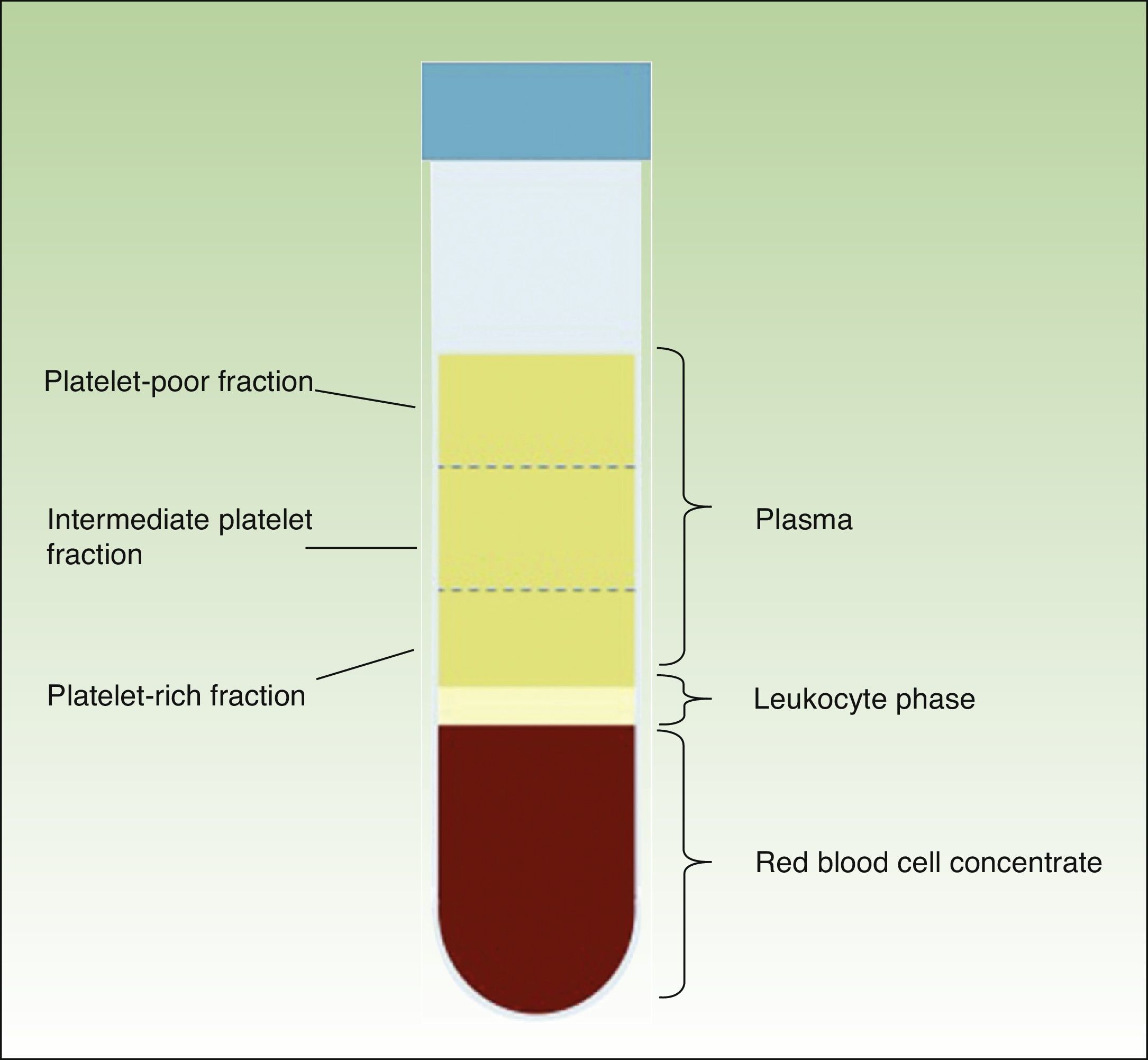
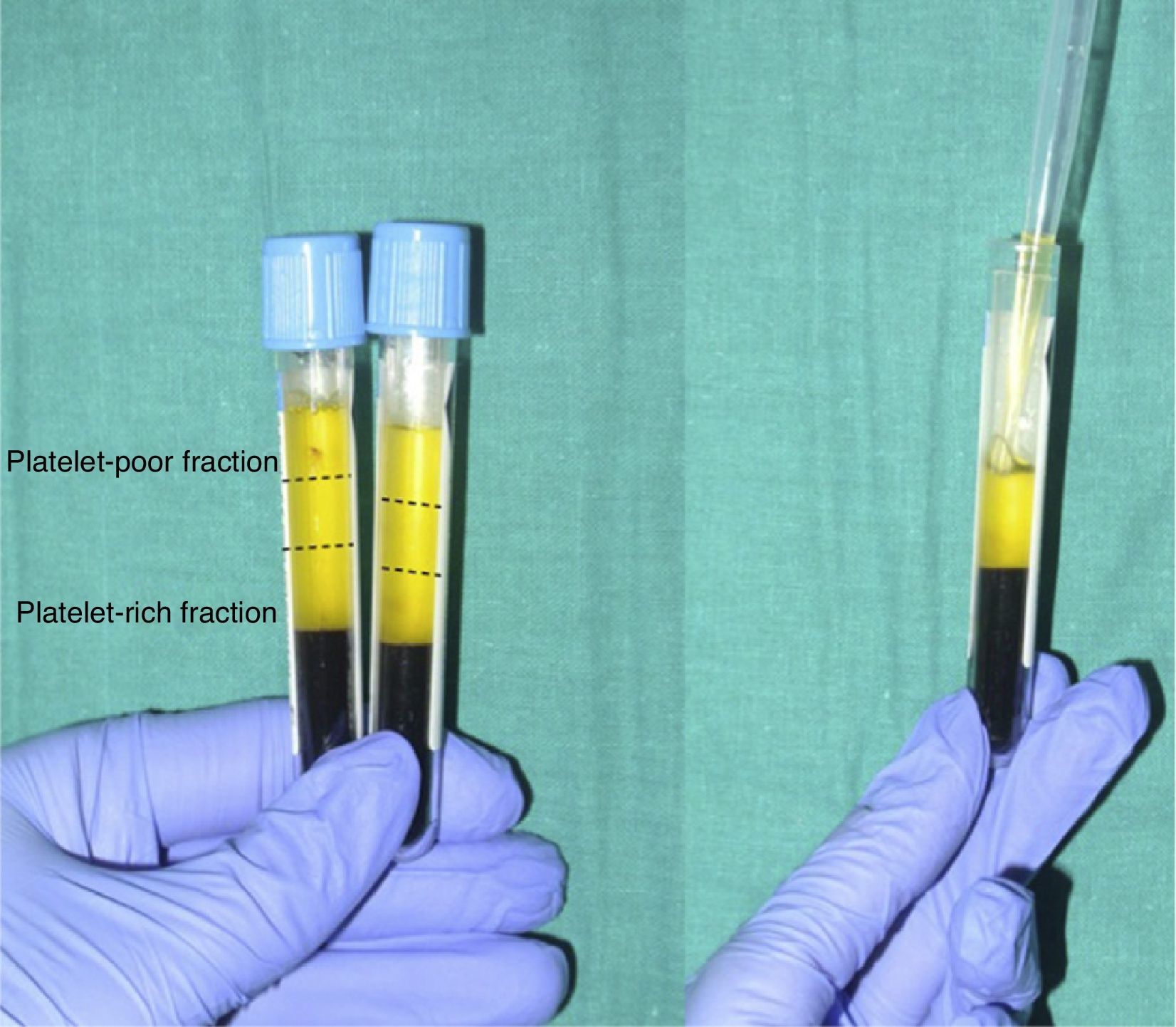
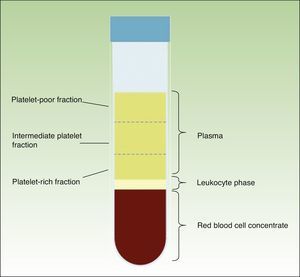
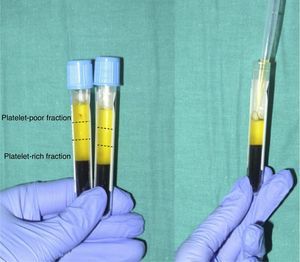
When the anticoagulated blood is centrifuged, 3 layers with different densities separate out: the lower layer, composed of red blood cells; the middle layer, composed of white blood cells and platelets; and the upper layer, composed of plasma. The plasma phase, in turn, can be subdivided into 3 fractions according to the number of platelets present. These fractions are, from most to least abundant: the platelet-poor fraction, the intermediate fraction with a medium concentration of platelets, and the platelet-rich fraction (Figure 2). This division of the plasma phase cannot be detected by eye, so the fraction is simply subdivided into the upper, lower, and middle thirds. Each fraction is separated into different sterile tubes by pipetting (Figure 3). The quality of the product obtained will depend on the skill and experience of the personnel who perform the pipetting. In order to achieve platelet degranulation and the subsequent release of the growth factors and other bioactive molecules, the lower fraction of the plasma phase has to be activated. The platelet-rich phase can be activated with different agents, with calcium chloride and thrombin being the most widely used.9,10 The product can be applied by injection or as a gel. In the former case the activated mix will be injected within 10minutes whereas in the latter case the technician will wait until a gel has formed. This normally requires heating or the addition of bioactive polymers.
Different in vitro assays have been developed to establish the cell and molecular content of the different commercial systems. Of note among the results obtained is the substantial interindividual variability and the lack of proportionality between platelet concentration and quantity or growth factors obtained with different methods.11 The clinical repercussions of these differences have still not been determined.
Safety of Application of Platelet-Rich PlasmaGiven the autologous nature of PRP, it is considered a safe product that by definition lacks the potential risk of disease transmission implicit in the use of blood products from donors. The systems that use bovine thrombin as activator are being phased out to avoid the development of coagulation disorders or secondary hypersensitivity. No evidence of the oncogenic potential of PRP suggested by some authors has been found.12 Growth factors, after binding to membrane receptors, activate intracellular signaling cascades that promote normal genetic expression regulated by different control mechanisms. To date no systemic effect has been demonstrated of growth factors released after local application of PRP.
With regard to the preparation conditions, the AEMPS has established some minimal quality requirements that have to be met by the prescribing physicians. A sterility test and product traceability are considered mandatory, and patient follow-up is necessary. In the case of methods for obtaining PRP manually from scratch, an inspection by the competent authority should be requested to assess the suitability of the facilities and the quality of production. When a commercial kit is used, the manufacturer instructions should be followed and a prior request is not necessary. The kit should, however, have the CE conformity mark granted for the specified use.4
Platelet-Rich Plasma and Skin UlcersChronic skin ulcer is characterized by extensive and deep loss of substance from the epidermis, dermis and sometimes deeper layers, with the lesion not healing in the expected time and with a limited tendency to heal. Such ulcers are associated with a prolonged inflammatory phase, with a greater presence of proinflammatory cytokines than acute ulcers. The most prevalent chronic skin ulcers in Spain are vascular ulcers on the legs, those occurring in diabetic foot, and pressure ulcers. The first study of the prevalence of chronic leg ulcers in Spain reported a figure of 0.16%.13 Despite correct diagnosis and treatment, even in chronic ulcer units, between 10% and 20% of these ulcers do not heal satisfactorily,14 with the resulting psychosocial impact and a substantial associated economic burden on the health services.15 Among the factors that have been implicated in a chronic course of lesions are the lack of growth factors, which are degraded in excess by bacterial or cell proteases, and deficient fibrin production. An effective intervention must therefore modify this environment that impedes healing3 and it is essential to induce the reparative phase of healing, and shorten the prior inflammatory phase.
The first clinical application of platelet-derived preparations was in chronic leg ulcers. The lesions were covered with collagen embedded in platelet proteins. With this product, known as platelet-derived wound healing formula (PDWHF), the formation of vascularized connective tissue was induced in these wounds.1,16 Since then, different platelet preparations have been tried, for application in solution, gel, or by injection. Reports correspond mainly to individual cases or case series,17–19 although pilot studies and clinical trials have also been conducted.20–28 The outcomes of the isolated cases and small series published are often spectacular, with a mean healing time of less than 12 weeks. These reports show a noteworthy variability in the size and etiology of the lesions, as well as the method for obtaining and applying PRP. With the use of manual preparations from scratch, the outcomes published are similar to those obtained in case series treated with PRP obtained using commercial kits.17 In a clinical trial of patients randomly assigned to topical treatment with PRP or conventional treatment (cleaning, debridement, and application of saline-soaked gauze dressing), a total of 14 ulcers were treated (64% venous ulcers, 29% pressure ulcers, and 7% other types of ulcer). After 8 weeks, the healed surface area in patients treated with PRP was significantly greater (72.94% [22.25%]) than in the control group (21.48% [33.56%]) (P<.05).28 However, in a recent meta-analysis, which included the aforementioned study and other clinical trials to give a total of 9 trials with 325 patients, the authors concluded that there were no differences between the PRP-treated group and the control group in terms of healing.29 Four of the trials included ulcers with mixed etiology,16,23,27,28 3 included venous ulcers,24–26 and the remaining 2 included leg ulcers in patients with diabetic foot.21,22 The mean duration of treatment was 12 weeks (range, 8-40 weeks). Only 1 of the studies had a low risk of bias23 and the outcome measures differed among the trials included. Seven of the trials used the proportion of completely healed ulcers, while 3 assessed total re-epithelized area, 2 assessed the percentage of the wound area healed, and 3 recorded wound complications. Statistically significant differences, in favor of the PRP-treated group, were only observed in the percentage of ulcer area cured. The authors concluded that further clinical trials with greater statistical power were needed to be able to confirm the real benefit of PRP in skin ulcers, given that the findings of their study are at odds with the good clinical response reported in numerous case reports. The negative finding could be due to the difficulty in performing a meta-analysis of studies that used the same product but with different methods of preparation. Furthermore, patients were enrolled with different inclusion criteria and there was a lack of uniformity in the outcome measures used. We should bear in mind that the inherent variability in the process for obtaining and applying the PRP might make it more difficult to design and conduct viable clinical studies. In addition to not knowing the ideal concentration of platelets, leukocytes, and growth factors, as well as the potential influence of exercise or drugs used prior to blood extraction, the role of the microenvironment and mechanical stimulation in lesions is not known. Likewise, factors which, alone, might influence cell differentiation or tissue repair (synergistically or antagonistically) are not known.
PRP can also been used as an adjuvant for improving the viability of grafts for the treatment of recalcitrant ulcers, with good results.30
Platelet-Rich Plasma and Skin AgeingAgeing is a progressive multifactorial process characteristic of the final stages of the life cycle, in which tissue and organic function of the organism decline, resulting in a lower level of adaptation to environmental changes.
More specifically, skin ageing leads to decreased vascularization, limited replenishment of cells and the intercellular matrix, skin appendage disorders, fat atrophy, and loss of muscle tone. In vivo studies have shown PRP exercises a bioregenerating action through stimulation of fibroblast proliferation, with an increase in antiinflammatory factors (HGF), angiogenic factors (VEGF), and proteins related to extracellular matrix remodeling such as procollagen I, hyaluronic acid, and tissue inhibitor of metallopeptidase 1 (TIMP-1).31,32 The main function of procollagen I is to increase dermal resistance to tension and stretching. Hyaluronic acid is found in the interstitial liquid that surrounds these collagen fibers, and has a lubricating action. Furthermore, as a result of its high water-retaining capacity, hyaluronic acid is considered a good hydrating agent that can increase skin firmness. TIMP-1, which inhibits metalloprotease activity, stabilizes the extracellular matrix. It is applied topically using a mask or by intradermal injections. The few studies that have assessed the clinical benefit of PRP in skin ageing used largely subjective outcome measures (for example, patient satisfaction and physician satisfaction through comparison of photographs).33 In a randomized clinical trial of 100 patients with obvious signs of skin ageing, the efficacy and safety of PRP compared to hyaluronic acid were assessed after 3 treatment sessions. Hydration and skin pH, change in wrinkle depth, and patient satisfaction were assessed in 2 follow-up visits at 3 and 6 months. The improvement in the outcome variables in the PRP-treated group was statistically significant.34 Some studies also suggest a beneficial role for PRP as an adjuvant for other rejuvenation techniques. Its use is becoming more commonplace after chemical or physical exfoliation or after laser resurfacing. One study showed how the technique was able to reduce erythema and accelerate healing in patients treated with ablative fractional carbon dioxide laser.35
Based on the benefits described for PRP in the improvement of signs of ageing, different preparations for topical application have been brought onto the market. Their formulation includes growth factors and soluble matrix proteins secreted by human dermal fibroblasts.
Another application of PRP is liposculpting, where it is used for optimizing free fatty acid grafts for subsequent infiltration. In vitro studies have shown a significant increase in the number of adipocytes. Clinical studies have shown that PRP can prolong the duration of the restorative environment compared to fatty infiltrations by themselves.36,37
Platelet-Rich Plasma and AlopeciaThe hair follicle cycle is responsible for regulating hair growth. Hair follicles transition from anagen (active phase) to catagen (involution due to apoptosis) and subsequently enter in telogen (rest phase). Many growth factors participate in the regulation of the follicular cycle, controlling the active phase and promoting catagen or telogen induction.38
The role of PRP in promoting hair survival and growth has been demonstrated both in vitro and in vivo.39,40 One study showed how it is possible to stimulate growth and increase hair density if the follicles are embedded in PRP prior to grafting.39 Although the specific mechanism of action of PRP on the hair follicle is still unknown, Li et al.40 found increased secretion of follicular growth factor 7 and β-catenin, with the subsequent proliferation of cells of the hair papilla and activation of the extracellular kinase- and Akt-dependent signaling pathways. The study showed that PRP injection in mice promoted an acceleration in the transition from telogen to anagen compared to the control group. In a pilot study, Kang et al.41 suggested that the potential benefits of interfollicular injection of a PRP preparation with CD34+ cells in the treatment of female- and male-pattern alopecia were due to their angiogenic action.
A randomized, double-blind clinical trial has been conducted in which the benefit of PRP application was assessed in 45 patients with alopecia areata. On comparing triamcinolone acetonide with placebo, a significant increase in repopulation was found along with a decrease in hair dystrophy and less pruritus and itching.42 In addition to stimulating cell proliferation, with an increase in Ki-67 in treated areas, the known anti-inflammatory action of PRP could help explain the good outcomes obtained in alopecia areata.
Platelet-Rich Plasma in Other ApplicationsGiven the proven activity of PRP in promoting tissue regeneration, various other applications for this product have been proposed.
The activity of PRP as a biological glue is the rationale for using it to improve the viability of skin flaps in recipient tissue beds. It is used in plastic surgery and dermatological surgery in processes that require the use of flaps to accelerate healing, improve sealing by eliminating dead space, decrease bleeding and the need for drainage and compression dressing, and reduce edema and postoperative pain.43
Lee et al.44 reported faster resolution of edema and erythema with the application of PRP after ablation with a carbon dioxide laser for the treatment of acne scars in 14 patients. The authors affirmed that there was a synergistic effect with PRP, with clinical improvement in the appearance of the scars.
Nicoli et al.45 reported a case of suppurative hidradenitis resistant to multiple treatments, with good response to the combination of surgery, PRP, and Hyalomatrix, a system that releases hyaluronic acid.
Final ConsiderationsPRP has been shown to be potentially beneficial as a bioregenerator in many applications in different fields of medicine, including dermatology. However, given the increasing number of cases published with good outcomes, the lack of well-designed studies to assess the efficacy of PRP in these different applications is noteworthy. In the aforementioned report by the AEMPS, the agency makes a call for robust clinical trials to provide stronger scientific evidence to support the indications for PRP.4 In the case of application in chronic skin ulcers, the protocolization and increasingly widespread use of PRP techniques in hospitals could have substantial impact on the quality of life of the patients and healthcare costs. In the Gregorio Marañón University Hospital in Madrid, Spain, we are achieving excellent outcomes using a manual technique from scratch to obtain the product in a small series of patients with chronic skin ulcers. After validation of the method, we intend to conduct an open-label randomized clinical trial to study the usefulness of PRP in healing chronic ulcers that do not respond to conventional treatment.
A deeper understanding of the biological principles of PRP and the molecular mechanisms implicated in tissue regeneration will help optimize the formulations and applications of PRP. More clinical studies are needed to support the efficacy of this safe and simple technique with multiple potential benefits and help establish it as a routine treatment with well-defined indications in dermatology.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Conde Montero E, Fernández Santos ME, Suárez Fernández R. Platelet-Rich Plasma: Applications in Dermatology. Actas Dermosifiliogr. 2015;106:104–111.