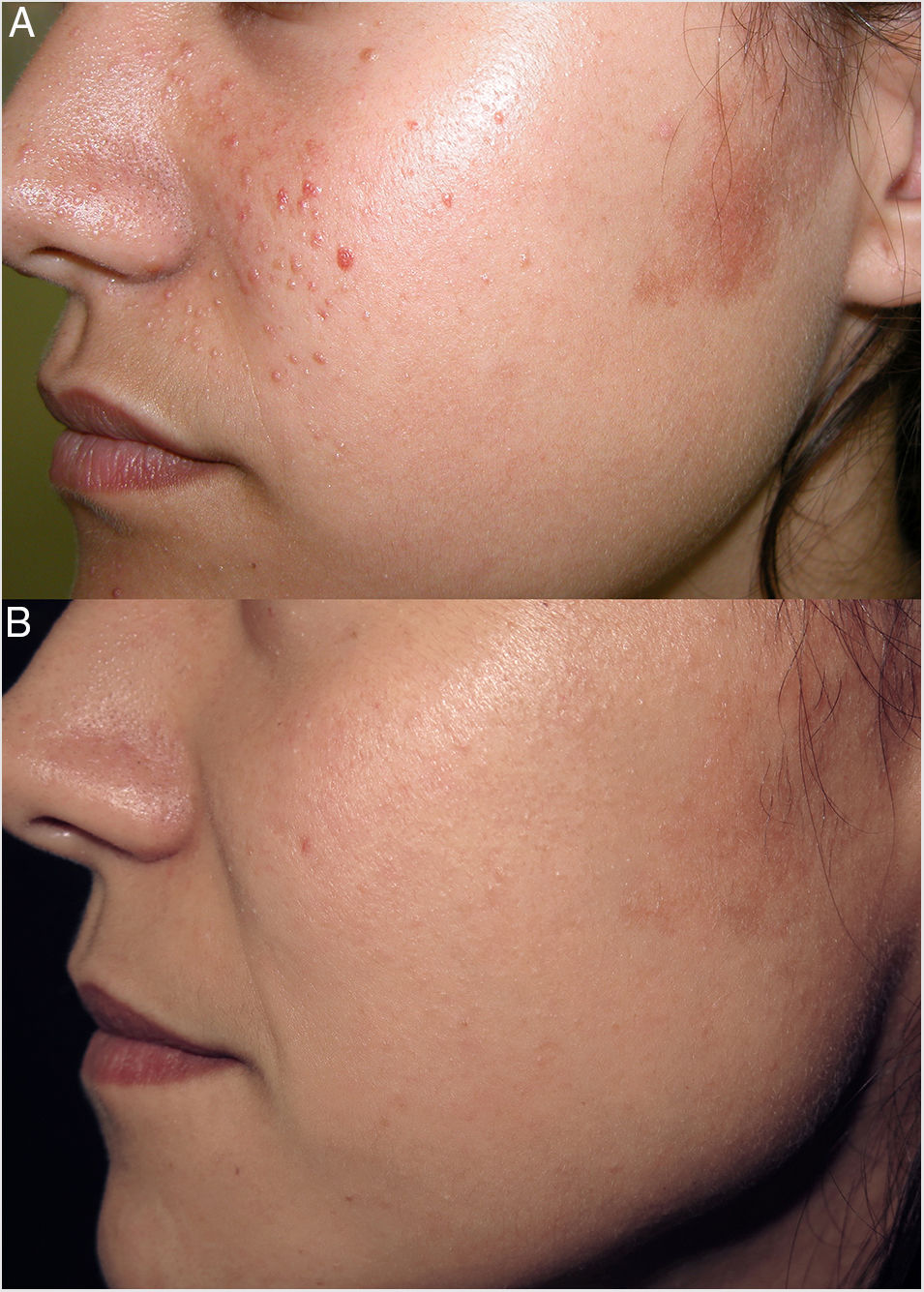
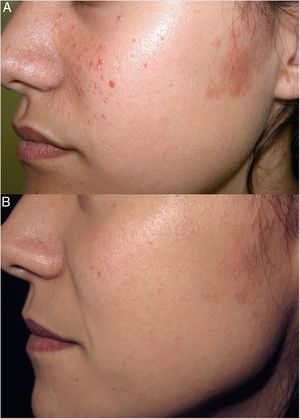
Rapamycin (sirolimus) is an inhibitor of mTOR. Topical rapamycin is effective for the treatment of facial angiofibroma and hypomelanotic macules in photoexposed areas in patients with tuberous sclerosis complex (TSC), and has shown variable efficacy in the treatment of cephalic plaque.
Our patient was a 24-year-old woman with a diagnosis of TSC who had undergone carbon dioxide (CO2) laser treatment of several angiofibromas. Two months after CO2 laser treatment, at 18 years of age, she began daily treatment of the remaining facial angiofibromas and of a cephalic plaque in the left preauricular region with a topical formulation of 0.2% rapamycin in liquid petrolatum (Fig. 1A). During the first 6 months of treatment the number of angiofibromas decreased markedly, and the preauricular cephalic plaque became flatter and less colored. The improvement observed in subsequent annual check-ups was less marked, albeit progressive and perceptible (Fig. 1B). The patient expressed a high degree of satisfaction with the treatment, experienced no adverse effects, and achieved excellent cosmetic results that allow her to mask the remaining lesions with makeup. In TSC patients, topical rapamycin could constitute a useful alternative to surgical or laser treatment of fibrous cephalic plaque.
Please cite this article as: Giacaman A, Martín-Santiago A. Placa cefálica en esclerosis tuberosa: tratamiento con rapamicina al 0,2%. Actas Dermosifiliogr. 2019;110:e13.