Psoriatic lesions affecting the scalp, nails, palms, and the soles of the feet are described as difficult-to-treat psoriasis and require specific management. Involvement of these sites often has a significant physical and emotional impact on the patient and the lesions are difficult to control with topical treatments owing to inadequate penetration of active ingredients and the poor cosmetic characteristics of the vehicles used. Consequently, when difficult-to-treat sites are involved, psoriasis can be considered severe even though the lesions are not extensive. Scant information is available about the use of biologic therapy in this setting, and published data generally comes from clinical trials of patients who also had moderate to severe extensive lesions or from small case series and isolated case reports. In this article we review the quality of the scientific evidence for the 4 biologic agents currently available in Spain (infliximab, etanercept, adalimumab, and ustekinumab) and report level i evidence for the use of biologics to treat nail psoriasis (level of recommendation A) and a somewhat lower level of evidence in the case of scalp involvement and palmoplantar psoriasis.
El término de psoriasis en localizaciones de difícil tratamiento se emplea para hacer referencia a la psoriasis localizada en el cuero cabelludo, las uñas, las palmas y las plantas y que requiere un manejo diferenciado. A menudo los pacientes presentan un importante impacto físico y emocional, unido a la dificultad para controlar adecuadamente sus lesiones con tratamientos tópicos, debido a una insuficiente penetración de los principios activos y la escasa cosmeticidad de los vehículos empleados. Esta circunstancia justifica que la psoriasis en estas localizaciones pueda ser considerada grave, a pesar de su extensión limitada. La experiencia con terapias biológicas en estas localizaciones es escasa, en general en el contexto de ensayos clínicos de formas extensas de psoriasis moderada y grave, junto con series limitadas o casos aislados. En el presente artículo se presenta la calidad de la evidencia científica para los 4 agentes biológicos disponibles en España (infliximab, etanercept, adalimumab y ustekinumab) siendo de nivel i en el caso de la psoriasis ungueal (nivel de recomendación A) y algo inferior en la psoriasis del cuero cabelludo y palmoplantar.
The Spanish Psoriasis Group of the Spanish Academy of Dermatology and Venereology (AEDV) has published an update of their evidence-based guidelines on the treatment of psoriasis with biologic agents.1 To complement those guidelines, this article reviews the scientific evidence available on the treatment of psoriasis in difficult-to-treat sites, such as the nails, scalp, palms and soles. As there is currently no evidence in the literature regarding the efficacy and safety of biologic therapy in the treatment of flexural and genital psoriasis, the treatment of these sites will not be discussed.
The term difficult-to-treat psoriasis has been used by several authors in recent years to refer to psoriasis (usually psoriasis vulgaris) localized to the scalp, palms/soles, and nails, which requires special attention because it is often associated with a significant physical and emotional impact on the patient, and sometimes even functional impairment.2–4 Many authors also include in this group psoriasis localized to areas of sensitive skin, such as the face, skin folds, and genitals.2,3,5 Psoriasis in difficult-to-treat sites also requires special treatment because topical medications are often ineffective and disagreeable to use so that systemic therapy is generally prescribed.2–5 In some patients, lesions at these sites are the only manifestation of the disease and they display a marked resistance to conventional therapies.
Very few controlled trials have evaluated the efficacy and safety of systemic therapy (classic or biologic) in the management of psoriasis in these sites. In general, the evidence available is obtained from subanalyses of trials involving patients with psoriasis and/or psoriatic arthritis (PsA) in which the involvement of the nails, scalp, palms or soles is also assessed. In addition to the usual tools for assessing the severity of psoriasis—the Psoriasis Area and Severity Index (PASI), the Body Surface Area (BSA), and the Physician Global Assessment (PGA)—several scales have been designed specifically to measure the severity of psoriasis affecting difficult-to-treat sites, such as the Nail Psoriasis Severity Index (NAPSI) and the modified target NAPSI in the case of nail psoriasis.6,7 The Psoriasis Scalp Severity Index (PSSI)8 and the Palmoplantar Pustular Psoriasis Area Severity Index (PPPASI)9 are both variants of the PASI. In order to assess the impact of psoriasis on the patient's quality of life, we have tools that measure the overall psychological and social impact of the disease; these include the Dermatology Life Quality Index (DLQI) as well as site-specific tools, such the Nail Psoriasis Quality of Life scale (NPQ10).10
Objectives and MethodsOur objective was to evaluate the quality of the available scientific evidence on the efficacy and safety of treatment with infliximab, etanercept, adalimumab, and ustekinumab in psoriasis affecting the nails, scalp, palms of the hands, and soles of the feet.
We performed a search of the medical literature in both English and Spanish by searching PubMed (MEDLINE) using the following search strategy: nail psoriasis AND/OR scalp psoriasis AND/OR palmoplantar psoriasis, followed each time by the name of each one of the different biologic treatments studied. The cut-off date for the search was September 7, 2013. The resulting draft document was submitted to the members of the Spanish Psoriasis Group, who collaborated in this review article.
Nail PsoriasisNail involvement in the course of psoriasis is found in between 50% and 80% of patients, and its incidence increases to as high as 90% in the life of an individual patient.11 A recent epidemiological study in Asturias of 661 patients with psoriasis reported that nail psoriasis was 13.5% more prevalent in men than in women. Furthermore, the group of patients with nail involvement had a more severe form of psoriasis, higher PASI scores, and a higher body mass index.12 Cases of isolated nail psoriasis have been reported,13 although nail involvement is usually preceded or accompanied by other skin and/or joint manifestations. In recent years, nail disease has been shown to be an early marker of enthesitis in PsA and an understanding of that phenomenon could help to elucidate the link between psoriasis and arthitis.14
Despite recent advances in topical therapies, nail psoriasis responds better to systemic therapy, especially in patients who have severe forms of the disease (NAPSI > 10).13,15,16
Guidelines for the treatment of nail psoriasis based on a review of the Cochrane Skin Group Specialised Register have recently been published.17 The review included 18 clinical studies involving a total of 1266 patients. After noting the poor quality of the studies, the authors concluded that the only treatments that resulted in significant nail improvement compared to placebo were infliximab, golimumab, superficial radiotherapy, Grenz rays, and electron beam.17
A retrospective study of 84 patients with moderate to severe psoriasis and/or PsA compared the effectiveness of classic systemic therapy (acitretin, ciclosporin, methotrexate, PUVA, and narrowband UV-B) with biologic therapy (efalizumab, infliximab, etanercept, and adalimumab) in the treatment of nail psoriasis at weeks 12, 24, and 48.18 One of the most interesting findings in that study was the correlation between PASI and NAPSI scores and the greater effectiveness of the biologic drugs, particularly infliximab and adalimumab at weeks 12 and 24. Ciclosporin was the most effective classic systemic treatment and only narrowband UV-B phototherapy was ineffective.
A prospective comparative study that evaluated the effectiveness of systemic therapy in nail psoriasis assessed outcomes in 87 patients, who were divided into 5 groups according to the treatment they received: methotrexate, acitretin, narrowband UV-B, biologic therapy, or no treatment (control group).19 At week 16, no significant differences in NAPSI values were found between patients receiving traditional systemic drugs and the control group. However, a significant decrease in NAPSI was detected when the authors compared the patients treated with biologic agents and the controls.
Anti-TNF α TherapiesInfliximabIn 2005, the multicenter, double-blind, placebo-controlled EXPRESS trial studied 378 patients with moderate to severe psoriasis.20,21 In that trial, patients treated with infliximab showed a significant improvement in mean NAPSI from week 10 onwards, with reductions as high as 57.2% at week 24. Moreover, the patients in whom skin lesions responded very well to treatment (a reduction in PASI >90%) showed a mean improvement of 80.3% in NAPSI at week 50.20 A retrospective analysis of the EXPRESS trial reported a mean improvement in NAPSI of 28.3% at week 10, 61.4% at week 24, and 67.8% at week 50.21
In 2 Japanese Phase III clinical trials involving a total of 90 patients, significant improvement was observed in nail psoriasis in the patients treated with infliximab.22
In a recently published prospective study, Saraceno et al.23 compared the efficacy of tumor necrosis factor (TNF) antagonists in nail psoriasis.23 In total, 60 patients were enrolled in 3 treatment groups (adalimumab, etanercept, and infliximab) and studied for 24 weeks. At week 14, efficacy (assessed as a reduction in NAPSI) was higher in the infliximab group compared to the other 2 agents. At week 24, all 3 drugs achieved a similar reduction in mean NAPSI.
In an uncontrolled open-label prospective study, Rigopoulos et al.25 evaluated the efficacy of infliximab in 18 patients with psoriasis and nail involvement. Significant improvement in nail disease was observed in most of the patients at week 14, with a reduction in mean NAPSI from a baseline of 55.8 to 29.8. After 6 months of treatment, the mean NAPSI had declined to 3.3.
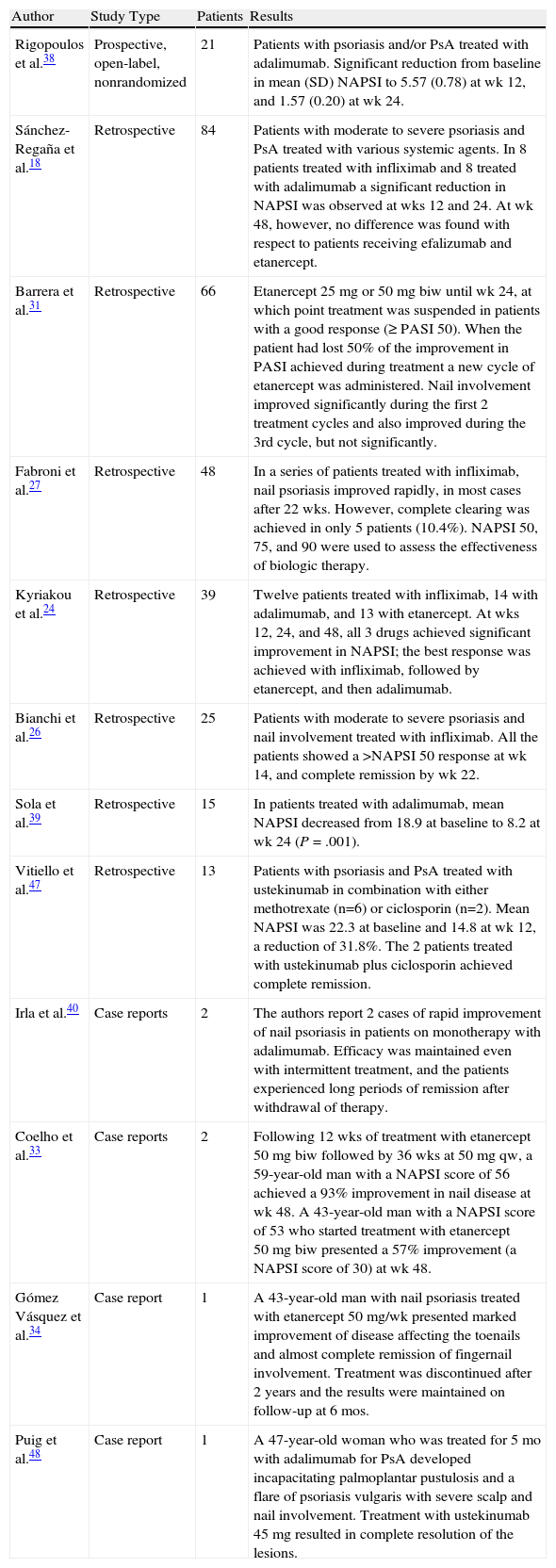
Table 1 shows the results of other studies in the literature involving patients with nail psoriasis who were treated with infliximab and other biologic agents.
Results of Other Studies in Nail Psoriasis.
| Author | Study Type | Patients | Results |
| Rigopoulos et al.38 | Prospective, open-label, nonrandomized | 21 | Patients with psoriasis and/or PsA treated with adalimumab. Significant reduction from baseline in mean (SD) NAPSI to 5.57 (0.78) at wk 12, and 1.57 (0.20) at wk 24. |
| Sánchez-Regaña et al.18 | Retrospective | 84 | Patients with moderate to severe psoriasis and PsA treated with various systemic agents. In 8 patients treated with infliximab and 8 treated with adalimumab a significant reduction in NAPSI was observed at wks 12 and 24. At wk 48, however, no difference was found with respect to patients receiving efalizumab and etanercept. |
| Barrera et al.31 | Retrospective | 66 | Etanercept 25 mg or 50 mg biw until wk 24, at which point treatment was suspended in patients with a good response (≥PASI 50). When the patient had lost 50% of the improvement in PASI achieved during treatment a new cycle of etanercept was administered. Nail involvement improved significantly during the first 2 treatment cycles and also improved during the 3rd cycle, but not significantly. |
| Fabroni et al.27 | Retrospective | 48 | In a series of patients treated with infliximab, nail psoriasis improved rapidly, in most cases after 22 wks. However, complete clearing was achieved in only 5 patients (10.4%). NAPSI 50, 75, and 90 were used to assess the effectiveness of biologic therapy. |
| Kyriakou et al.24 | Retrospective | 39 | Twelve patients treated with infliximab, 14 with adalimumab, and 13 with etanercept. At wks 12, 24, and 48, all 3 drugs achieved significant improvement in NAPSI; the best response was achieved with infliximab, followed by etanercept, and then adalimumab. |
| Bianchi et al.26 | Retrospective | 25 | Patients with moderate to severe psoriasis and nail involvement treated with infliximab. All the patients showed a >NAPSI 50 response at wk 14, and complete remission by wk 22. |
| Sola et al.39 | Retrospective | 15 | In patients treated with adalimumab, mean NAPSI decreased from 18.9 at baseline to 8.2 at wk 24 (P=.001). |
| Vitiello et al.47 | Retrospective | 13 | Patients with psoriasis and PsA treated with ustekinumab in combination with either methotrexate (n=6) or ciclosporin (n=2). Mean NAPSI was 22.3 at baseline and 14.8 at wk 12, a reduction of 31.8%. The 2 patients treated with ustekinumab plus ciclosporin achieved complete remission. |
| Irla et al.40 | Case reports | 2 | The authors report 2 cases of rapid improvement of nail psoriasis in patients on monotherapy with adalimumab. Efficacy was maintained even with intermittent treatment, and the patients experienced long periods of remission after withdrawal of therapy. |
| Coelho et al.33 | Case reports | 2 | Following 12 wks of treatment with etanercept 50 mg biw followed by 36 wks at 50 mg qw, a 59-year-old man with a NAPSI score of 56 achieved a 93% improvement in nail disease at wk 48. A 43-year-old man with a NAPSI score of 53 who started treatment with etanercept 50 mg biw presented a 57% improvement (a NAPSI score of 30) at wk 48. |
| Gómez Vásquez et al.34 | Case report | 1 | A 43-year-old man with nail psoriasis treated with etanercept 50 mg/wk presented marked improvement of disease affecting the toenails and almost complete remission of fingernail involvement. Treatment was discontinued after 2 years and the results were maintained on follow-up at 6 mos. |
| Puig et al.48 | Case report | 1 | A 47-year-old woman who was treated for 5 mo with adalimumab for PsA developed incapacitating palmoplantar pustulosis and a flare of psoriasis vulgaris with severe scalp and nail involvement. Treatment with ustekinumab 45 mg resulted in complete resolution of the lesions. |
Abbreviations: NAPSI, Nail Psoriasis Severity Index; PASI, Psoriasis Area Severity Index; PsA, psoriatic arthritis.
Some authors consider that a specific profile of candidates for treatment with infliximab could be proposed because of the characteristics of this agent.28 The profile would include patients with severe plaque psoriasis associated with severe nail involvement and/or PsA.
EtanerceptThe authors of the CRYSTEL study evaluated the effect of treatment with etanercept on patients with moderate to severe psoriasis and determined the prevalence of nail and joint symptoms and their impact on the patients’ quality of life.29 Of the 711 patients evaluated, who were randomized to receive either continuous (352) or paused (359) etanercept therapy for 54 weeks, 79% had nail psoriasis at the start of the study. Patients with nail psoriasis showed significant improvement at the end of the study, as evidenced by a 51% reduction in mean NAPSI.
In a recent randomized clinical trial (the NAIL study), Ortonne et al.30 analyzed the efficacy and safety of etanercept in the treatment of nail psoriasis in patients with moderate to severe psoriasis. The study included patients with nail psoriasis who had previously failed to respond to treatment with at least 1 systemic drug. Patients were randomized to receive either etanercept 50 mg twice weekly for 12 weeks followed by 50 mg weekly for 12 weeks or etanercept 50 mg for 24 weeks. At week 24, the authors observed a significant correlation between NAPSI and PASI scores and a significant decrease in the NAPSI score for the target fingernail, similar in both groups.
A prospective, multicenter, observational study was carried out in several Dutch hospitals to evaluate the effectiveness of TNF blockers in the treatment of juvenile PsA.32 The study included 18 patients. Nail pitting was observed in 39% of the patients at baseline. Seventeen patients were treated with etanercept and 1 with adalimumab. In most of the patients, the authors reported a good response in the case of psoriatic joint symptoms but not for skin and nail disease; 4 patients even developed de novo psoriasis during treatment.
AdalimumabThe authors of a post hoc analysis of Phase IIIb of the BELIEVE clinical trial investigated the effect of PsA on the efficacy of adalimumab and the response to treatment of other markers of psoriasis burden, such as skin lesions, nail involvement, pain, pruritus, and quality of life.35 In that study, treatment with adalimumab resulted in considerable clinical improvement in skin and nail disease.
The REACH randomized controlled trial included 72 patients.36 Of the 31 patients who had nail psoriasis, a 50% reduction in NAPSI (NAPSI 50) was achieved in a higher percentage of the adalimumab-treated patients (56.5%) than the controls (12.5%) at week 16.
An uncontrolled, prospective study conducted in 2010 of 442 patients with PsA assessed treatment with adalimumab 40 mg administered subcutaneously every other week for 12 weeks.37 The mean NAPSI of the 164 patients with severe PsA and nail psoriasis was reduced by 57% at 12 weeks.
Adverse Effects on Nails During Treatment With Anti-TNF-α TherapyIn recent years, several studies have reported that the prevalence of onychomycosis is greater in patients with nail psoriasis than in the healthy population, with rates ranging from 4.6% to 47.9%.18,41 Both dermatophyte and non-dermatophyte yeasts have been isolated.11,18,41 Al-Mutairi et al.42 studied the rate of onychomycosis in patients with nail psoriasis randomized to receive biologic therapy with etanercept, infliximab, or adalimumab for 24 weeks. In total 315 patients were studied and the results were compared with controls. Onychomycosis in association with nail psoriasis was more frequent in men and in patients treated with infliximab (33%). The prevalences for the other groups were 15.45% for etanercept, 13.33% for adalimumab, and 13.89% for the controls.
It has also been reported that patients being treated with TNF-α inhibitors for diseases other than psoriasis have developed psoriasis, nail psoriasis, and/or palmoplantar pustular psoriasis, and sometimes all 3 conditions. In a series of 120 patients, all 3 TNF-α inhibitors available on the market triggered this type of cutaneous lesions: infliximab (n = 63), etanercept (n = 37), and adalimumab (n = 26).43 Psoriasis was the most common (n = 73), followed by palmoplantar pustular psoriasis (n = 37), and nail psoriasis (n = 6). In 75 patients, the induced psoriasis was de novo, while in 24 the reaction was an exacerbation or aggravation of preexisting psoriatic disease.
UstekinumabIn the PHOENIX 1 randomized phase III trial, treatment with ustekinumab 45 mg achieved a mean reduction in NAPSI of 25% at week 12 and 50% at week 24.44
The results have recently been published of a double-blind, placebo-controlled Phase II/III trial designed to assess the safety and efficacy of ustekinumab in Japanese patients with moderate to severe psoriasis.45 In total, 158 patients were randomized to receive ustekinumab or placebo with cross-over to ustekinumab at week 12. A significant reduction in NAPSI was observed after 12 weeks of treatment and maintained through week 72.
In 2011, the authors of an open-label, uncontrolled study in Greece evaluated the effectiveness of ustekinumab in nail psoriasis, prospectively enrolling 27 patients who were treated with ustekinumab according to the regimen specified in the SPC.46 Disease severity was assessed using the Onychomycosis Quality of Life and NAPSI scores. The authors reported significant reductions in both indices at 4, 16, 28, and 40 weeks.
Scalp PsoriasisThe scalp is affected in 80% of patients with psoriasis49 and is often the first site of onset. Scalp involvement is frequently associated with a marked deterioration in quality of life because it affects the patient's self esteem, social life, and lifestyle. In fact, 57% of patients with scalp psoriasis report psychological problems and social stress caused by the itching and scaling that characterizes this condition.49,50
Despite the broad range of topical therapies currently available, scalp psoriasis is often resistant to these treatments. While there is abundant literature on the use of biologic therapies in psoriasis in general, scant evidence is available that relates specifically to their use in scalp psoriasis.51–53
Anti-TNF α TherapiesInfliximabIn a subanalysis of the results of 3 double-blind, randomized trials in which infliximab was used to treat moderate to severe psoriasis, Menter et al.54 examined its effectiveness in different areas of the body. A total of 1462 patients had been included in these 3 trials (EXPRESS, EXPRESS II, and SPIRIT). Following treatment with infliximab for 10 weeks, an improvement in PASI response similar to the overall value was observed for all 4 sites studied (head-neck, upper limbs, trunk, and lower limbs). At 10 weeks, the percentage of patients with at least a PASI 75 response or at least a PASI 90 response in the infliximab group vs placebo was as follows: 85.5% vs 22% (≥ 75%) and 68.9% vs 12% (≥ 90%) in the SPIRIT trial; 84.8% vs 12.5% (≥ 75%) and 73.1% vs 8.3% (≥ 90%) in the EXPRESS study; and 79% vs 10% (≥ 75%) and 66.6% vs 5.5% (≥ 90%) in the EXPRESS II trial.
EtanerceptThe authors of an open-label, randomized study that compared the efficacy and safety of continuous (n = 1272) vs interrupted (n = 1274) treatment with etanercept reported a 58% reduction in PGA for scalp psoriasis at 12 weeks in both groups.55 At week 24, the efficacy observed in scalp psoriasis was greater in the continuous treatment group.
In a randomized, double-blind, placebo-controlled trial, Bagel et al.53 evaluated the efficacy of etanercept in patients with moderate to severe psoriasis and scalp involvement. At 12 weeks, etanercept 50 mg administered twice weekly achieved an 87% reduction in PSSI, as compared to a 20% reduction in the placebo group. Continued treatment with etanercept 50 mg once a week maintained the response in scalp psoriasis through week 24.
In a randomized, double-blind, placebo-controlled study, Tyring et al.56 assessed patients with psoriasis and scalp involvement receiving treatment with etanercept using patient-reported outcomes relating to pruritus, pain, health-related quality of life, depression, emotional stress, and patient satisfaction with treatment. At 12 weeks, they observed a significant difference between the outcomes reported by the patients treated with etanercept and the controls.
AdalimumabThe multicenter BELIEVE randomized clinical trial assessed the efficacy of combining therapy with adalimumab—at the dosage specified in the SPC—and topical treatment (calcipotriol 50 μg/g plus betamethasone 0.5 mg/g) in patients with moderate to severe psoriasis who had previously had an inadequate response to systemic therapies.35 Although the study protocol did not call for the application of topical treatment on the scalp or nails, the PSSI and NAPSI were used to assess the response to adalimumab at weeks 0, 8, and 16. In total, 663 of the 730 patients in the study (91.3%) had scalp psoriasis (PSSI > 0). After 16 weeks of treatment the mean reduction in PSSI score was 77.2%.
The authors of a prospective observational study in Japan that compared the therapeutic efficacy of adalimumab and infliximab in the treatment of psoriasis vulgaris also assessed the impact of both drugs on scalp involvement.57 They studied 21 patients, some of whom were on concomitant topical treatment with vitamin D analogues. Patients were treated with infliximab or adalimumab, according to their preference. At week 16, the authors observed a significantly lower PSSI response rate in the group receiving adalimumab (54.5%) compared to the infliximab-treated group (90%).
In a retrospective study, Sola et al reported significant improvement in scalp psoriasis at 24 weeks following treatment with adalimumab at the dosage regimen specified in the SPC.39
UstekinumabTwo cases have been reported of moderate to severe psoriasis in which ustekinumab administered at the doses specified in the SPC resulted in rapid improvement of scalp lesions at week 8, with a high level of adherence to treatment and a positive impact on quality-of-life.58
Palmoplantar PsoriasisPalmoplantar psoriasis is a variant that causes significant morbidity and has a major impact on patients’ quality-of-life. Patients develop cracks, hyperkeratosis, and sometimes pustules on the hands and/or feet. The symptoms cause pain and itching, lead to impaired mobility, and have a negative effect on the patient's social life.59,60 Topical therapy is generally more unsatisfactory in these sites than in others and disease tends to be recalcitrant.61 The literature contains few studies specifically targeting patients with palmoplantar psoriasis, and indeed patients with this condition are often specifically excluded from clinical trials. The evidence relating to these sites is so scant that it is impossible to differentiate between the treatment of palmoplantar psoriasis and that of palmoplantar pustular psoriasis. Since some authors have reported paradoxical induction of palmoplantar pustular psoriasis with the use or discontinuation of anti-TNF therapy, the use of these drugs in the treatment of palmoplantar psoriasis has been questioned.62
Anti-TNF α TherapiesInfliximabA multicenter randomized controlled trial assessed the efficacy and safety of treatment with infliximab in 24 patients with nonpustular palmoplantar psoriasis.63 At week 14, significant improvement was observed in the treatment group (50% reduction in the mean surface area of the affected palms and soles as compared to an increase of 15% in the group receiving placebo). However, the study did not achieve its primary endpoint since at week 14 only 33% of patients had achieved a 75% reduction on the modified PPPASI used. The authors attributed these results to the small size of the sample and the fact that the patients included had very low baseline scores.
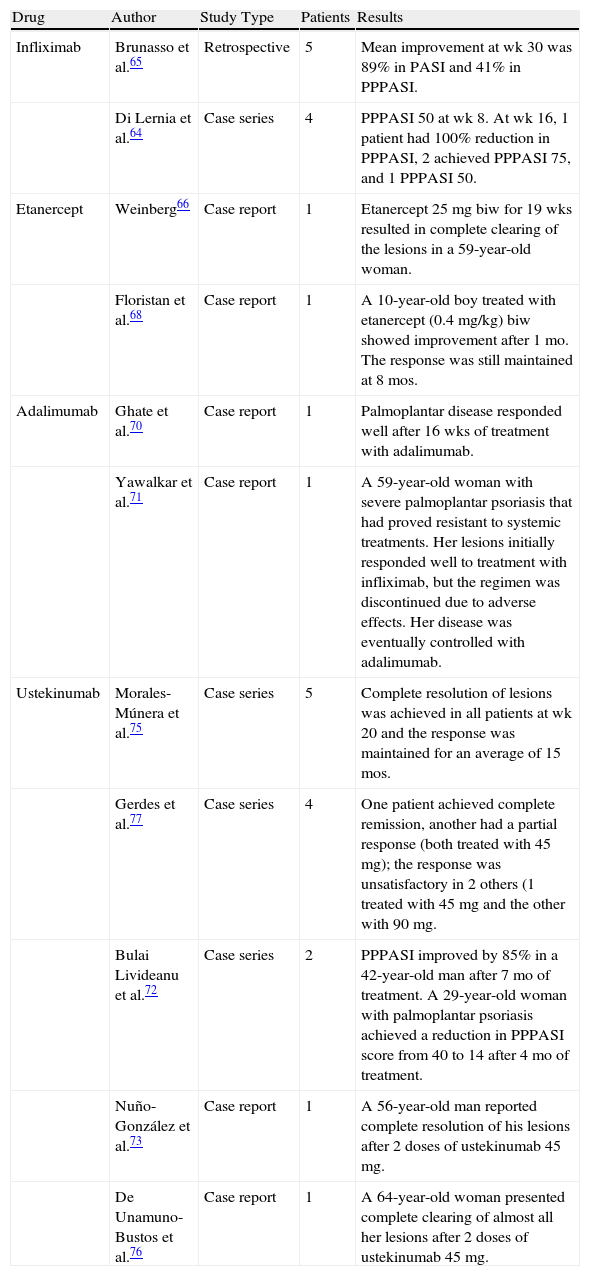
Table 2 shows the results of other studies and case reports in the literature in which palmoplantar psoriasis was treated with infliximab or other biologic agents.
Palmoplantar Psoriasis: Retrospective Studies, Case Series, and Case Reports in the Literature.
| Drug | Author | Study Type | Patients | Results |
| Infliximab | Brunasso et al.65 | Retrospective | 5 | Mean improvement at wk 30 was 89% in PASI and 41% in PPPASI. |
| Di Lernia et al.64 | Case series | 4 | PPPASI 50 at wk 8. At wk 16, 1 patient had 100% reduction in PPPASI, 2 achieved PPPASI 75, and 1 PPPASI 50. | |
| Etanercept | Weinberg66 | Case report | 1 | Etanercept 25mg biw for 19 wks resulted in complete clearing of the lesions in a 59-year-old woman. |
| Floristan et al.68 | Case report | 1 | A 10-year-old boy treated with etanercept (0.4 mg/kg) biw showed improvement after 1 mo. The response was still maintained at 8 mos. | |
| Adalimumab | Ghate et al.70 | Case report | 1 | Palmoplantar disease responded well after 16 wks of treatment with adalimumab. |
| Yawalkar et al.71 | Case report | 1 | A 59-year-old woman with severe palmoplantar psoriasis that had proved resistant to systemic treatments. Her lesions initially responded well to treatment with infliximab, but the regimen was discontinued due to adverse effects. Her disease was eventually controlled with adalimumab. | |
| Ustekinumab | Morales-Múnera et al.75 | Case series | 5 | Complete resolution of lesions was achieved in all patients at wk 20 and the response was maintained for an average of 15 mos. |
| Gerdes et al.77 | Case series | 4 | One patient achieved complete remission, another had a partial response (both treated with 45mg); the response was unsatisfactory in 2 others (1 treated with 45 mg and the other with 90 mg. | |
| Bulai Livideanu et al.72 | Case series | 2 | PPPASI improved by 85% in a 42-year-old man after 7 mo of treatment. A 29-year-old woman with palmoplantar psoriasis achieved a reduction in PPPASI score from 40 to 14 after 4 mo of treatment. | |
| Nuño-González et al.73 | Case report | 1 | A 56-year-old man reported complete resolution of his lesions after 2 doses of ustekinumab 45 mg. | |
| De Unamuno-Bustos et al.76 | Case report | 1 | A 64-year-old woman presented complete clearing of almost all her lesions after 2 doses of ustekinumab 45 mg. |
Abbreviations: PASI, Psoriasis Area Severity Index; PPPASI, Palmoplantar Pustular Psoriasis Area Severity Index.
In a randomized controlled trial, Bissonnette et al.67 analyzed the efficacy of etanercept in the treatment of palmoplantar pustulosis. Fifteen patients were randomized (2:1) to receive either etanercept 50 mg or placebo twice weekly for 3 months, and then all the patients in the study were treated with etanercept 50 mg twice weekly for a further 3 months. At 24 weeks, a statistically significant reduction was observed in the PPPASI in the patients treated with etanercept throughout the whole study but not in the crossover group (P = .038, n = 10).
AdalimumabThe chief objective of the randomized controlled REACH trial was to assess the efficacy of adalimumab in the treatment of palmoplantar psoriasis.36 The study enrolled 72 patients, who were randomized (2:1) to receive either adalimumab (49) or placebo (23). The authors found that in the group of patients treated with adalimumab those who achieved at least a NAPSI 50 response also improved their score on the PGA scale relating to clearing of hands and/or feet.
An open-label study by Richetta et al.69 included 11 patients treated with adalimumab 40 mg administered subcutaneously every other week. Of these, 54% (6/11) improved by at least 1 point on the PGA scale, and 8 of them also showed significant improvement on the DLQI scale at week 12.
UstekinumabIn an open-label study, Au et al.74 assessed treatment with ustekinumab administered according to the SPC in patients whose palmoplantar psoriasis had proven refractory to treatment with topical corticosteroids. Twelve patients improved by at least 2 points on the PGA scale for palms and soles, but complete resolution was achieved by only 7. Those authors also reported that 6 of the 9 patients who weighed over 100 kg and were treated with 90-mg doses achieved complete remission at 16 weeks. However, only 1 of the 11 patients weighing under 100 kg and treated with a 45-mg dose achieved clinical clearance at 16 weeks. In view of these results, the authors suggested that 90 mg could be the effective dose level for ustekinumab in the treatment of palmoplantar psoriasis.
Adverse Effects Affecting the Scalp, Palms and Soles During Biologic TherapyAs reported above in the case of nails, outbreaks of psoriasiform lesions on the scalp, palms and soles during biologic therapy have been reported.
Some 15 cases of scalp alopecia associated with psoriasiform lesions have been reported during anti-TNF therapy. Two of the cases reported were forms of scarring alopecia, and the rest were clinically similar to alopecia areata or psoriatic alopecia.78,79 Biopsy results revealed psoriasiform epidermal features—not found in alopecia areata—and dermal infiltrates of plasma cells and eosinophils uncharacteristic of psoriatic alopecia. Based on these clinical and histologic findings, Doyle et al.80 have suggested that this could be a new cause of non-scarring alopecia. In general, the condition responded well to topical treatment. In severe cases, anti-TNF therapy should be withdrawn because of the risk of scarring alopecia.78–80Paradoxical adverse effects on the palms and soles are more common during treatment with TNF inhibitors. This phenomenon has been studied by Joyau et al.,81 who performed a comprehensive search of the literature and the French pharmacovigilance register to gather data on paradoxical reactions occurring during the course of biologic therapy. They found 57 cases in the register (January 2002-September 2009) and 184 cases in the literature. The lesions most often reported were palmoplantar pustules, which accounted for 33.3% of the cases of paradoxical eruption in the French registry and 42.9% of those identified in the literature. Most of the patients affected were women aged between 40 and 50 years. It is now considered that these forms of psoriasis that appear during anti-TNF therapy are de novo forms and not exacerbations of preexisting psoriasis.81
A single case has been reported of exacerbation of palmoplantar inflammatory changes, fissures, and hyperkeratosis after 6 weeks of treatment with ustekinumab in a 35-year-old woman with ankylosing spondylitis who had previously developed a pustular erythematous eruption in the same palmoplantar area during treatment with infliximab.82
ConclusionsThe use of biologic therapy in patients with psoriasis affecting difficult-to-treat sites (nails, scalp, palms, and soles) is indicated when the disease fulfills any of the following conditions: not controlled with topical or conventional systemic treatment; extensive disease (NAPSI > 10); rapid worsening; subjective perception of severity (DLQI > 10); or associated PsA. (Table 3).
Recommendations: Eligibility Criteria for Biologic Treatment in Difficult-to-Treat Sites (Nails, Scalp, Palms and Soles).
| Treatment with biologic drugs in patients with psoriasis in difficult-to-treat sites is indicated when the disease fulfils any of the following conditions: |
| a) disease uncontrolled with topical or conventional systemic treatment |
| b) extensive lesions: NAPSI > 10 |
| c) rapid worsening of disease |
| d) subjective perception of severity on the part of the patient: DLQI >10 |
| e) Concomitant psoriatic joint disease |
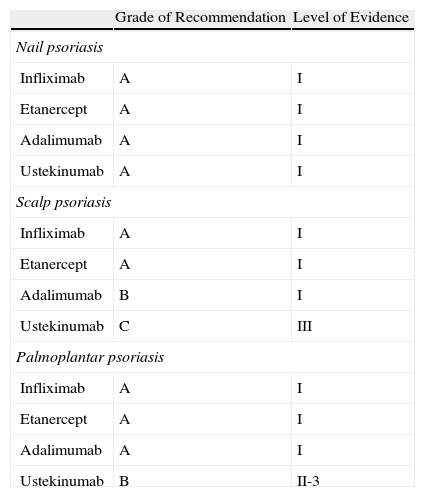
For nail psoriasis, the level of evidence is I and the grade of recommendation is A for all 4 biologic agents evaluated (infliximab, etanercept, adalimumab, and ustekinumab). In the case of scalp psoriasis and palmoplantar psoriasis, the level of evidence is somewhat lower (Table 4).
Grade of Recommendation and Quality of Evidence for Biologic Agents in the Treatment of Psoriasis in Difficult-to-Treat Sites.
| Grade of Recommendation | Level of Evidence | |
| Nail psoriasis | ||
| Infliximab | A | I |
| Etanercept | A | I |
| Adalimumab | A | I |
| Ustekinumab | A | I |
| Scalp psoriasis | ||
| Infliximab | A | I |
| Etanercept | A | I |
| Adalimumab | B | I |
| Ustekinumab | C | III |
| Palmoplantar psoriasis | ||
| Infliximab | A | I |
| Etanercept | A | I |
| Adalimumab | A | I |
| Ustekinumab | B | II-3 |
Manuel Sánchez-Regaña, Isabel Belinchón, José Manuel Carrascosa, Carlos Ferrándiz, David Vidal, Ricardo Ruiz and Eduardo Fonseca have participated in clinical trials, acted as consultants, and/or have received lecture fees or grants to attend training events from one or more of the following pharmaceutical companies: Abbvie (formerly Abbott), Janssen, MSD, and Pfizer.
Esteban Daudén has received or is currently receiving grants, funding, or honoraria in respect of diverse activities (advisory board membership, consultancy work, research, participation in clinical trials, and lectures) from the following pharmaceutical companies: Abbvie (Abbott), Amgen, Astellas, Biogen, Centocor Ortho Biotech Inc., Galderma, Glaxo, Janssen-Cilag, Leo Pharma, MSD, Pfizer, Novartis, Stiefel and Celgene.
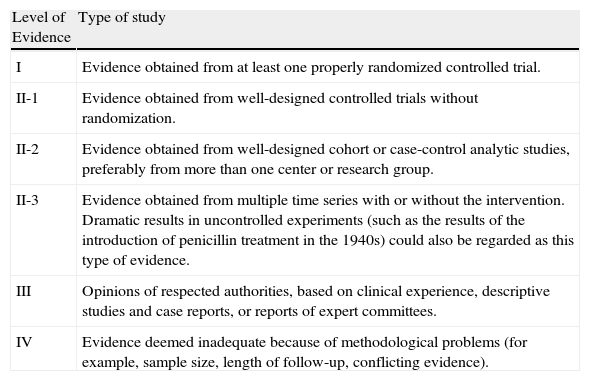
| Level of Evidence | Type of study |
| I | Evidence obtained from at least one properly randomized controlled trial. |
| II-1 | Evidence obtained from well-designed controlled trials without randomization. |
| II-2 | Evidence obtained from well-designed cohort or case-control analytic studies, preferably from more than one center or research group. |
| II-3 | Evidence obtained from multiple time series with or without the intervention. Dramatic results in uncontrolled experiments (such as the results of the introduction of penicillin treatment in the 1940s) could also be regarded as this type of evidence. |
| III | Opinions of respected authorities, based on clinical experience, descriptive studies and case reports, or reports of expert committees. |
| IV | Evidence deemed inadequate because of methodological problems (for example, sample size, length of follow-up, conflicting evidence). |
Source: Harris et al.83
Grade of Recommendation and Quality of Evidence for Treatment with Biologic Agents for Psoriasis in Difficult-to-Treat Sites
| Grade of Recommendation | Definition |
| A | Strongly recommended (good evidence that the intervention is effective and that the benefits substantially outweigh harms) |
| B | Recommended (at least fair evidence that the intervention is effective and the benefits outweigh harms) |
| C | No Recommendation (at least fair evidence that the intervention is effective, but concludes that the balance of benefits and harms is too close to justify a general recommendation) |
| D | Not recommended (at least fair evidence that the intervention is ineffective or that harms outweigh benefits |
| E | Insufficient Evidence to Make a Recommendation (evidence that the intervention is effective is lacking, of poor quality, or conflicting and the balance of benefits and harms cannot be determined) |
Please cite this article as: Sánchez-Regaña M, Aldunce M, Belinchón I, Ribera M, Lafuente-Urrez R, Carrascosa J, et al. Directrices del grupo español de psoriasis (GEP) basadas en la evidencia para el uso de medicamentos biológicos en pacientes con psoriasis en localizaciones de difícil tratamiento (uñas, cuero cabelludo, palmas y plantas). Actas Dermosifiliogr. 2014;105:923–934.