Topical interventions for rosacea are often used to relieve local symptoms. However, currently, there are few articles to systematically analyze the efficacy profile of topical drugs for rosacea. This study aimed to investigate the efficacy profile of widely used topical drugs. To acquire appropriate information from related literature, we looked into 4 databases. Efficacy was appraised with the Investigator Global Assessment, Clinician's Erythema Assessment, Patient's Self-Assessment and Subject Self-Assessment of Rosacea Facial Redness scales. Treatment-emergent adverse events and dermal tolerability were also recorded. According to 21 randomized controlled trials included, a total of 6 topical drugs including minocycline, ivermectin, azelaic acid, metronidazole, brimonidine and oxymetazoline were reported. These drugs are well-tolerated and safe. Ivermectin is more effective than azelaic acid and metronidazole. Azelaic acid has a better efficacy profile than metronidazole according to included studies. Minocycline turned out to be effective improving the symptoms of rosacea. Brimonidine and oxymetazoline both have significant effects on reducing facial redness.
La intervención local de la rosácea se utiliza generalmente para aliviar los síntomas locales. Sin embargo, hasta ahora, pocos artículos han analizado sistemáticamente la eficacia de los medicamentos locales en el tratamiento de la rosácea. El objetivo de este estudio es investigar la eficacia de los medicamentos locales de uso común. Para obtener la información adecuada de la literatura relevante, recuperamos cuatro bases de datos. La eficacia se evalúa mediante la evaluación general del investigador, la evaluación del eritema del clínico, la autoevaluación del paciente y la autoevaluación de la escala de enrojecimiento facial por rosácea del sujeto. También se registraron eventos adversos y tolerancia cutánea durante el tratamiento. Según los 21 ensayos aleatorizados controlados incluidos, hay seis fármacos tópicos: minociclina, ivermectina, ácido azelaico, metronidazol, bromonidina e hidroximetazolina. Estos medicamentos tienen una buena tolerancia y seguridad. La ivermectina es más eficaz que el ácido azelaico y el metronidazol. Según el estudio incluido, el ácido azelaico es mejor que el metronidazol. La minociclina puede mejorar eficazmente los síntomas de la rosácea. Tanto la bromonidina como la hidroximetazolina tienen un efecto significativo en la reducción del enrojecimiento facial.
Rosacea is a common chronic inflammatory skin disease that leads to flushing, redness, erythematous papules and pustules on the face1 and can affect the life quality and mental health of patients to some extent. Rosacea is generally categorized into 4 main subtypes based on its morphological features: erythematotelangiectatic, papulopustular, phymatous, and ocular.2 However, the exact pathogenesis of rosacea remains unclear and the clinical signs of different patients are complicated. There is a large No. of patients suffering from rosacea. In 2018, Gether L, et al. reported that approximately 5.46% of the adult population was affected by rosacea based on published information.3
Currently, there are various treatment options for rosacea including topical (e.g., metronidazole gel and azelaic acid gel) and systematic interventions (e.g., oral antibiotics and isotretinoin) and laser or light-based therapy. Although, it has been reported that pulsed dye light and intense pulsed light have a similar effect on reducing of facial erythema of rosacea,4 more studies are still needed. Topical drugs are the first-line therapy for mild-to-moderate rosacea.1 A systematic treatment or combination therapy should be considered to alleviate mild-to-moderate papulopustular rosacea.5
Topical drugs are often used to relieve the local symptoms and have gained more attention. There are many types of topical drugs which have been proven effective to treat rosacea. However, few articles have systematically analyzed the efficacy profile of topical drugs for rosacea so far. Our research tried to update the information of the curative effect of several topical drugs for rosacea. Based on former studies, we intended to evaluate the efficacy profile of topical drugs for rosacea by analyzing existing studies and comparing the incidence rate of adverse reactions.
Material and methodsData sources and searchesTwo writers conducted an independent search by December 2nd, 2024. Using the search phrases “rosacea AND topical”, we looked into 4 different databases: PubMed, Embase, Web of Science, and the Cochrane Library. Retrieval was not restricted by language.
Inclusion and exclusion criteriaThe following were the study inclusion criteria: (1) For studies: only randomized controlled trials (RCTs). (2) For subjects: clinical diagnosis of rosacea established by compatible history and physical examination. (3) For the experimental group: topical drugs were used to treat individuals from the experimental group. There are no limitations on how the control group is treated. The following were the exclusion criteria: (1) comments, reviews, letters, case reports or abstracts from conference proceedings; (2) repetitive studies; (3) articles lacking relevant data; and (4) articles not involving human subjects.
Outcome measuresThe primary terminal points to assess the efficacy profile were the proportion and number of individuals achieving “success” (defined as IGA≤1 in a 5-point system and IGA≤2 in a 7-point system), proportion and number of individuals achieving a 2-grade or greater decrease from baseline on both the CEA and the PSA in the last recorded treatment, proportion and number of individuals achieving a 2-grade or greater decrease from baseline on both the CEA and the SSA in the last recorded treatment. Additionally, the secondary outcome indicators recorded in the study were treatment-emergent adverse events (TEAEs) and cutaneous tolerance.
Data extraction and quality assessmentDatabases were independently looked into by 2 different writers using the inclusion and exclusion criteria. Arbitration would be used by a third author to settle the dispute. The author, the publication year, the nation, the interventions, the number and percentage of patients who achieved IGA success, the number and percentage of patients who saw a decrease of one or more grades from baseline on the CEA and PSA, the number and percentage of patients who saw a decrease of one or more grades from baseline on the CEA and SSA, TEAEs, and dermal tolerability were all taken from the article. The risk of bias from each study was evaluated using the Cochrane Reviewers’ Handbook standards as a guide.
Data analysis and synthesisWe synthetized data using the Review Manager software (RevMan 5.3.5) to conduct the meta-analysis. Binary data was extracted from each study for 2 groups to evaluate the efficacy profile of several widely used local drugs. Furthermore, a classification table was developed to determine the relative risk (RR, 95%CI) to obtain an aggregated overall estimate. Statistical testing of I2 and the Chi-square were used to check heterogeneity across studies. I2 values<50% show low heterogeneity; between 50% and 75%, substantial heterogeneity; and >75%, high heterogeneity. For the Chi-square test, the statistical correlation p-value represents the statistical significance of heterogeneity. In the presence of significant heterogeneity (I2>50%), a random effect model was used for analytical purposes. Otherwise, the fixed effect model was used.
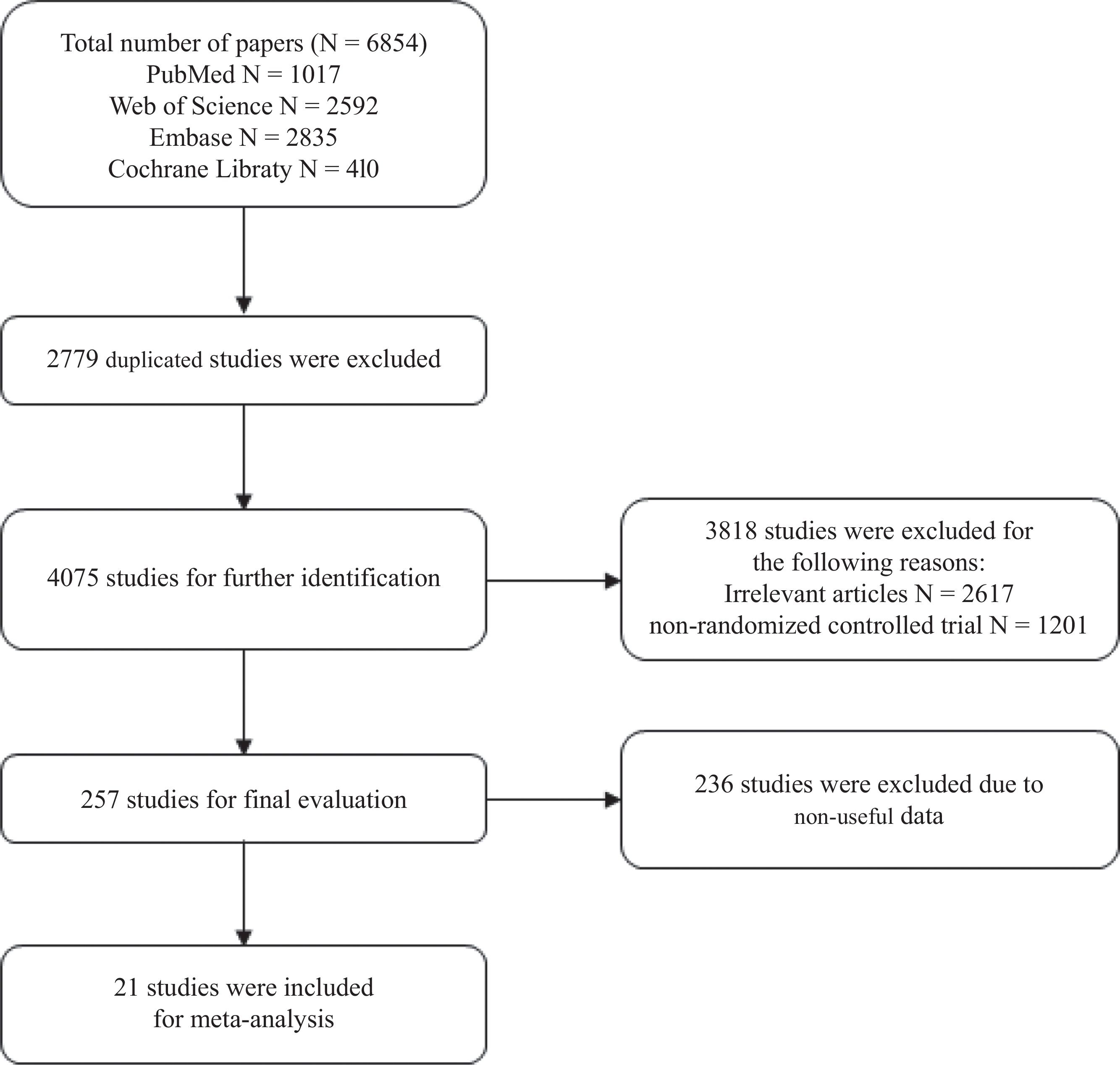
ResultsLiterature searchUsing the search terms, we found a total of 6854 articles. After removing duplicates, 4075 articles remained. After browsing titles and abstracts of these articles, 2819 unrelated articles were removed, and 999 articles were excluded as non-randomized controlled trials. Ultimately, after excluding 257 articles that did not have useful data, a total of a total of 21 articles were included in the meta-analysis. Fig. 1 shows the literature screening process.
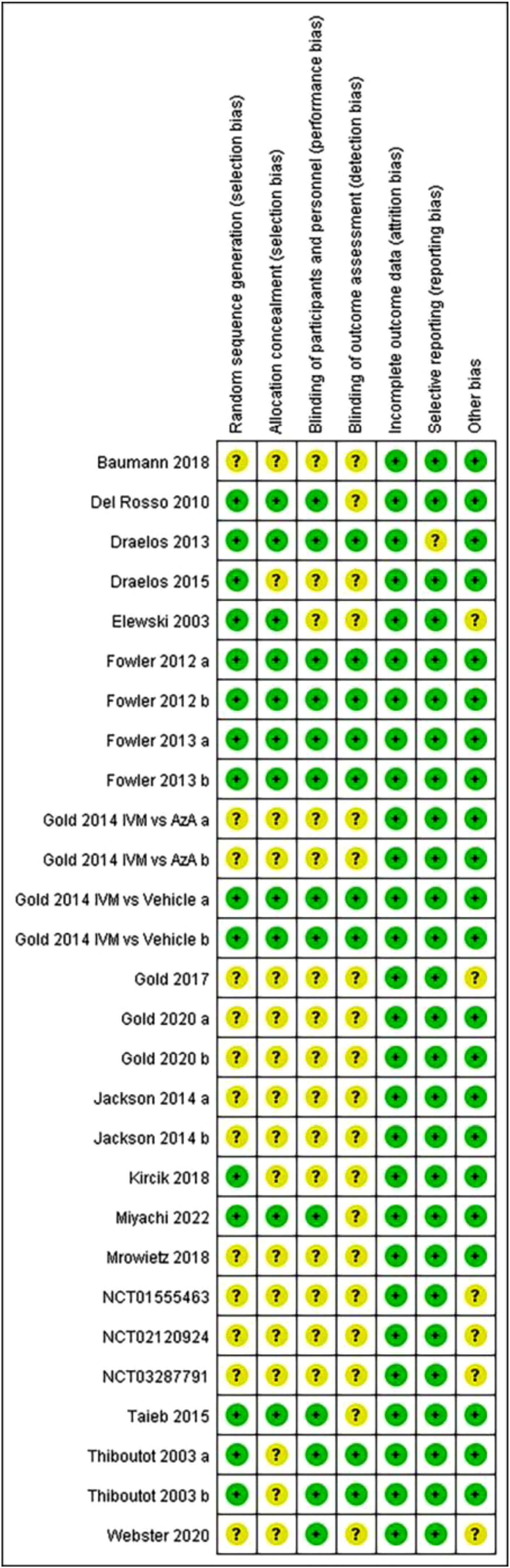
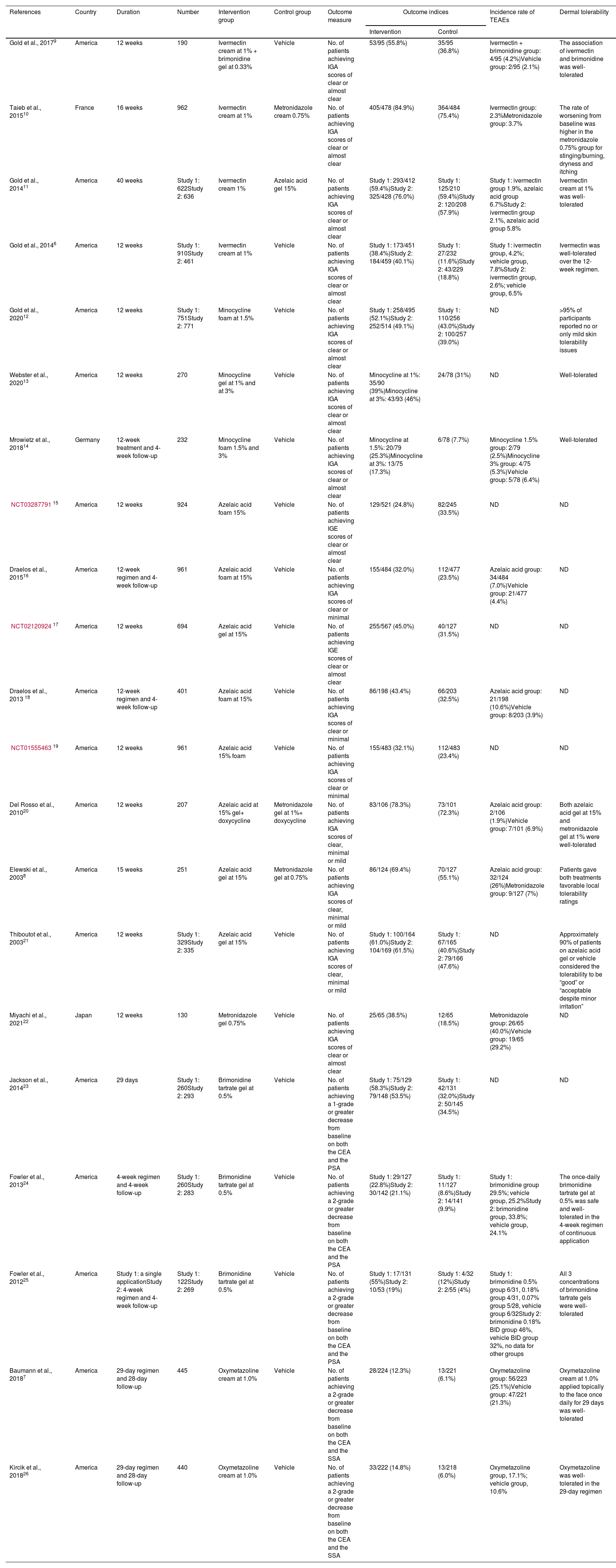
Study characteristics and risk of bias assessmentAll 21 articles included in the meta-analysis are in English. These articles came from 4 different countries (18 from the United States, 1 from France, 1 from Japan and 1 from Germany). Table 1 shows more details. A summary of risk of bias is shown in Fig. 2. Six studies had a low risk of bias while the other 22 studies were considered to have unclear risk of bias.
Characteristics of included studies.
| References | Country | Duration | Number | Intervention group | Control group | Outcome measure | Outcome indices | Incidence rate of TEAEs | Dermal tolerability | |
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||||
| Gold et al., 20179 | America | 12 weeks | 190 | Ivermectin cream at 1% + brimonidine gel at 0.33% | Vehicle | No. of patients achieving IGA scores of clear or almost clear | 53/95 (55.8%) | 35/95 (36.8%) | Ivermectin + brimonidine group: 4/95 (4.2%)Vehicle group: 2/95 (2.1%) | The association of ivermectin and brimonidine was well-tolerated |
| Taieb et al., 201510 | France | 16 weeks | 962 | Ivermectin cream at 1% | Metronidazole cream 0.75% | No. of patients achieving IGA scores of clear or almost clear | 405/478 (84.9%) | 364/484 (75.4%) | Ivermectin group: 2.3%Metronidazole group: 3.7% | The rate of worsening from baseline was higher in the metronidazole 0.75% group for stinging/burning, dryness and itching |
| Gold et al., 201411 | America | 40 weeks | Study 1: 622Study 2: 636 | Ivermectin cream 1% | Azelaic acid gel 15% | No. of patients achieving IGA scores of clear or almost clear | Study 1: 293/412 (59.4%)Study 2: 325/428 (76.0%) | Study 1: 125/210 (59.4%)Study 2: 120/208 (57.9%) | Study 1: ivermectin group 1.9%, azelaic acid group 6.7%Study 2: ivermectin group 2.1%, azelaic acid group 5.8% | Ivermectin cream at 1% was well-tolerated |
| Gold et al., 20146 | America | 12 weeks | Study 1: 910Study 2: 461 | Ivermectin cream at 1% | Vehicle | No. of patients achieving IGA scores of clear or almost clear | Study 1: 173/451 (38.4%)Study 2: 184/459 (40.1%) | Study 1: 27/232 (11.6%)Study 2: 43/229 (18.8%) | Study 1: ivermectin group, 4.2%; vehicle group, 7.8%Study 2: ivermectin group, 2.6%; vehicle group, 6.5% | Ivermectin was well-tolerated over the 12-week regimen. |
| Gold et al., 202012 | America | 12 weeks | Study 1: 751Study 2: 771 | Minocycline foam at 1.5% | Vehicle | No. of patients achieving IGA scores of clear or almost clear | Study 1: 258/495 (52.1%)Study 2: 252/514 (49.1%) | Study 1: 110/256 (43.0%)Study 2: 100/257 (39.0%) | ND | >95% of participants reported no or only mild skin tolerability issues |
| Webster et al., 202013 | America | 12 weeks | 270 | Minocycline gel at 1% and at 3% | Vehicle | No. of patients achieving IGA scores of clear or almost clear | Minocycline at 1%: 35/90 (39%)Minocycline at 3%: 43/93 (46%) | 24/78 (31%) | ND | Well-tolerated |
| Mrowietz et al., 201814 | Germany | 12-week treatment and 4-week follow-up | 232 | Minocycline foam 1.5% and 3% | Vehicle | No. of patients achieving IGA scores of clear or almost clear | Minocycline at 1.5%: 20/79 (25.3%)Minocycline at 3%: 13/75 (17.3%) | 6/78 (7.7%) | Minocycline 1.5% group: 2/79 (2.5%)Minocycline 3% group: 4/75 (5.3%)Vehicle group: 5/78 (6.4%) | Well-tolerated |
| NCT0328779115 | America | 12 weeks | 924 | Azelaic acid foam 15% | Vehicle | No. of patients achieving IGE scores of clear or almost clear | 129/521 (24.8%) | 82/245 (33.5%) | ND | ND |
| Draelos et al., 201516 | America | 12-week regimen and 4-week follow-up | 961 | Azelaic acid foam at 15% | Vehicle | No. of patients achieving IGA scores of clear or minimal | 155/484 (32.0%) | 112/477 (23.5%) | Azelaic acid group: 34/484 (7.0%)Vehicle group: 21/477 (4.4%) | ND |
| NCT0212092417 | America | 12 weeks | 694 | Azelaic acid gel at 15% | Vehicle | No. of patients achieving IGE scores of clear or almost clear | 255/567 (45.0%) | 40/127 (31.5%) | ND | ND |
| Draelos et al., 2013 18 | America | 12-week regimen and 4-week follow-up | 401 | Azelaic acid foam at 15% | Vehicle | No. of patients achieving IGA scores of clear or minimal | 86/198 (43.4%) | 66/203 (32.5%) | Azelaic acid group: 21/198 (10.6%)Vehicle group: 8/203 (3.9%) | ND |
| NCT0155546319 | America | 12 weeks | 961 | Azelaic acid 15% foam | Vehicle | No. of patients achieving IGA scores of clear or minimal | 155/483 (32.1%) | 112/483 (23.4%) | ND | ND |
| Del Rosso et al., 201020 | America | 12 weeks | 207 | Azelaic acid at 15% gel+ doxycycline | Metronidazole gel at 1%+ doxycycline | No. of patients achieving IGA scores of clear, minimal or mild | 83/106 (78.3%) | 73/101 (72.3%) | Azelaic acid group: 2/106 (1.9%)Vehicle group: 7/101 (6.9%) | Both azelaic acid gel at 15% and metronidazole gel at 1% were well-tolerated |
| Elewski et al., 20038 | America | 15 weeks | 251 | Azelaic acid gel at 15% | Metronidazole gel at 0.75% | No. of patients achieving IGA scores of clear, minimal or mild | 86/124 (69.4%) | 70/127 (55.1%) | Azelaic acid group: 32/124 (26%)Metronidazole group: 9/127 (7%) | Patients gave both treatments favorable local tolerability ratings |
| Thiboutot et al., 200321 | America | 12 weeks | Study 1: 329Study 2: 335 | Azelaic acid gel at 15% | Vehicle | No. of patients achieving IGA scores of clear, minimal or mild | Study 1: 100/164 (61.0%)Study 2: 104/169 (61.5%) | Study 1: 67/165 (40.6%)Study 2: 79/166 (47.6%) | ND | Approximately 90% of patients on azelaic acid gel or vehicle considered the tolerability to be “good” or “acceptable despite minor irritation” |
| Miyachi et al., 202122 | Japan | 12 weeks | 130 | Metronidazole gel 0.75% | Vehicle | No. of patients achieving IGA scores of clear or almost clear | 25/65 (38.5%) | 12/65 (18.5%) | Metronidazole group: 26/65 (40.0%)Vehicle group: 19/65 (29.2%) | ND |
| Jackson et al., 201423 | America | 29 days | Study 1: 260Study 2: 293 | Brimonidine tartrate gel at 0.5% | Vehicle | No. of patients achieving a 1-grade or greater decrease from baseline on both the CEA and the PSA | Study 1: 75/129 (58.3%)Study 2: 79/148 (53.5%) | Study 1: 42/131 (32.0%)Study 2: 50/145 (34.5%) | ND | ND |
| Fowler et al., 201324 | America | 4-week regimen and 4-week follow-up | Study 1: 260Study 2: 283 | Brimonidine tartrate gel at 0.5% | Vehicle | No. of patients achieving a 2-grade or greater decrease from baseline on both the CEA and the PSA | Study 1: 29/127 (22.8%)Study 2: 30/142 (21.1%) | Study 1: 11/127 (8.6%)Study 2: 14/141 (9.9%) | Study 1: brimonidine group 29.5%; vehicle group, 25.2%Study 2: brimonidine group, 33.8%; vehicle group, 24.1% | The once-daily brimonidine tartrate gel at 0.5% was safe and well-tolerated in the 4-week regimen of continuous application |
| Fowler et al., 201225 | America | Study 1: a single applicationStudy 2: 4-week regimen and 4-week follow-up | Study 1: 122Study 2: 269 | Brimonidine tartrate gel at 0.5% | Vehicle | No. of patients achieving a 2-grade or greater decrease from baseline on both the CEA and the PSA | Study 1: 17/131 (55%)Study 2: 10/53 (19%) | Study 1: 4/32 (12%)Study 2: 2/55 (4%) | Study 1: brimonidine 0.5% group 6/31, 0.18% group 4/31, 0.07% group 5/28, vehicle group 6/32Study 2: brimonidine 0.18% BID group 46%, vehicle BID group 32%, no data for other groups | All 3 concentrations of brimonidine tartrate gels were well-tolerated |
| Baumann et al., 20187 | America | 29-day regimen and 28-day follow-up | 445 | Oxymetazoline cream at 1.0% | Vehicle | No. of patients achieving a 2-grade or greater decrease from baseline on both the CEA and the SSA | 28/224 (12.3%) | 13/221 (6.1%) | Oxymetazoline group: 56/223 (25.1%)Vehicle group: 47/221 (21.3%) | Oxymetazoline cream at 1.0% applied topically to the face once daily for 29 days was well-tolerated |
| Kircik et al., 201826 | America | 29-day regimen and 28-day follow-up | 440 | Oxymetazoline cream at 1.0% | Vehicle | No. of patients achieving a 2-grade or greater decrease from baseline on both the CEA and the SSA | 33/222 (14.8%) | 13/218 (6.0%) | Oxymetazoline group, 17.1%; vehicle group, 10.6% | Oxymetazoline was well-tolerated in the 29-day regimen |
ND, non-disclosed; IGE, Investigator Global Evaluation (same as IGA).
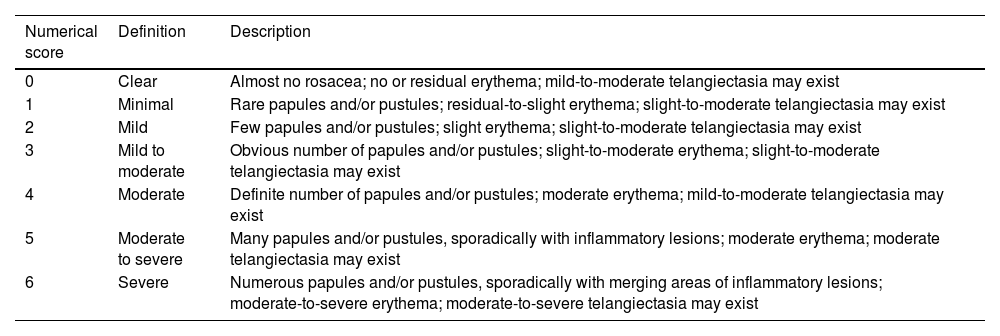
IGA (0–6).8
| Numerical score | Definition | Description |
|---|---|---|
| 0 | Clear | Almost no rosacea; no or residual erythema; mild-to-moderate telangiectasia may exist |
| 1 | Minimal | Rare papules and/or pustules; residual-to-slight erythema; slight-to-moderate telangiectasia may exist |
| 2 | Mild | Few papules and/or pustules; slight erythema; slight-to-moderate telangiectasia may exist |
| 3 | Mild to moderate | Obvious number of papules and/or pustules; slight-to-moderate erythema; slight-to-moderate telangiectasia may exist |
| 4 | Moderate | Definite number of papules and/or pustules; moderate erythema; mild-to-moderate telangiectasia may exist |
| 5 | Moderate to severe | Many papules and/or pustules, sporadically with inflammatory lesions; moderate erythema; moderate telangiectasia may exist |
| 6 | Severe | Numerous papules and/or pustules, sporadically with merging areas of inflammatory lesions; moderate-to-severe erythema; moderate-to-severe telangiectasia may exist |
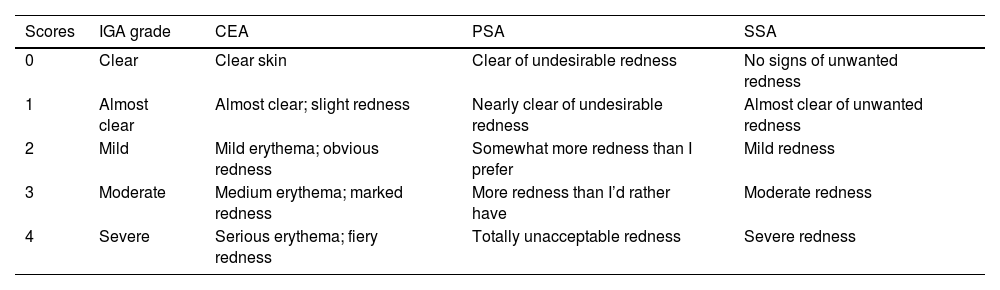
Investigator Global Assessment (IGA) (0–4), Clinician's Erythema Assessment (CEA), Patient's Self-Assessment (PSA) and Subject Self-Assessment of Rosacea Facial Redness (SSA) Scales.6,7
| Scores | IGA grade | CEA | PSA | SSA |
|---|---|---|---|---|
| 0 | Clear | Clear skin | Clear of undesirable redness | No signs of unwanted redness |
| 1 | Almost clear | Almost clear; slight redness | Nearly clear of undesirable redness | Almost clear of unwanted redness |
| 2 | Mild | Mild erythema; obvious redness | Somewhat more redness than I prefer | Mild redness |
| 3 | Moderate | Medium erythema; marked redness | More redness than I’d rather have | Moderate redness |
| 4 | Severe | Serious erythema; fiery redness | Totally unacceptable redness | Severe redness |
According to the articles included, 6 topical drugs for rosacea were identified whose efficacy profile can be analyzed, including ivermectin, minocycline, azelaic acid, metronidazole, brimonidine and oxymetazoline. The results of the meta-analysis and forest plot are shown in the following figures.
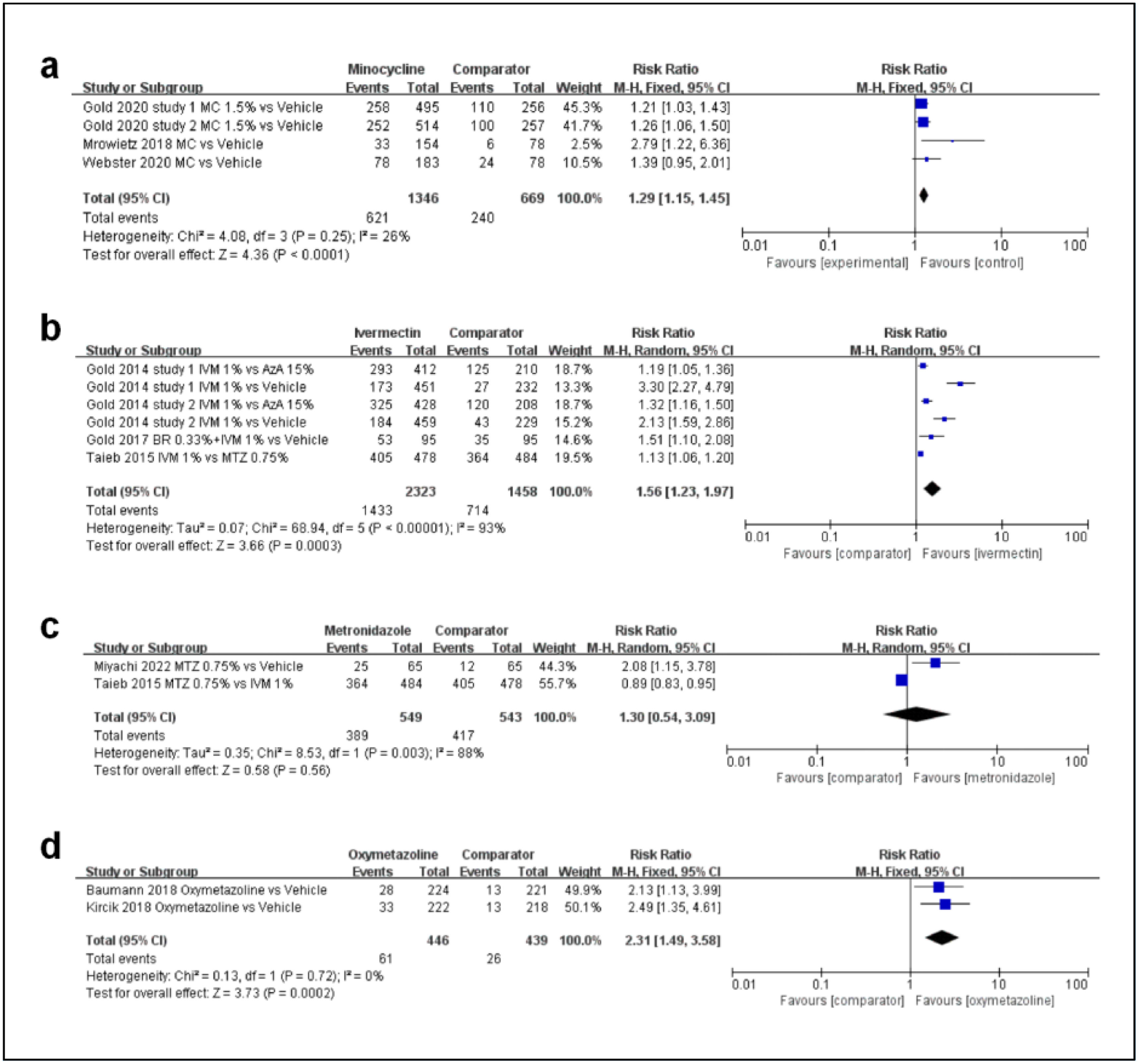
First, regarding the efficacy profile of minocycline, as shown in Fig. 3a, a total of 4 studies were included. There was a statistically significant difference between the minocycline group and vehicles (MD, 1.29; 95%CI, 1.15–1.45; p<0.00001).
Second, regarding the efficacy profile ivermectin, as shown in Fig. 3b, a total of 4 studies were included. There was a statistically significant difference between the ivermectin group and the comparator (MD, 1.56; 95%CI, 1.23–1.97; p=0.0003).
Third, regarding the efficacy profile metronidazole, as shown in Fig. 3c, a total of 2 studies were included. The meta-analysis estimated that there was no statistically significant difference in the rate of participants achieving IGA “success” (IGA≤1) between the metronidazole group at 0.75% and the comparator group (MD, 1.30; 95%CI, 0.54–3.09; p=0.56).
Fourth, regarding the efficacy profile oxymetazoline, as shown in Fig. 3d, a total of 2 studies were included. Oxymetazoline showed a statistically significant difference in the rate of participants achieving a 2-grade or greater decrease from baseline on both the CEA and the SSA (MD, 2.31; 95%CI, 1.49–3.58; p=0.0002).
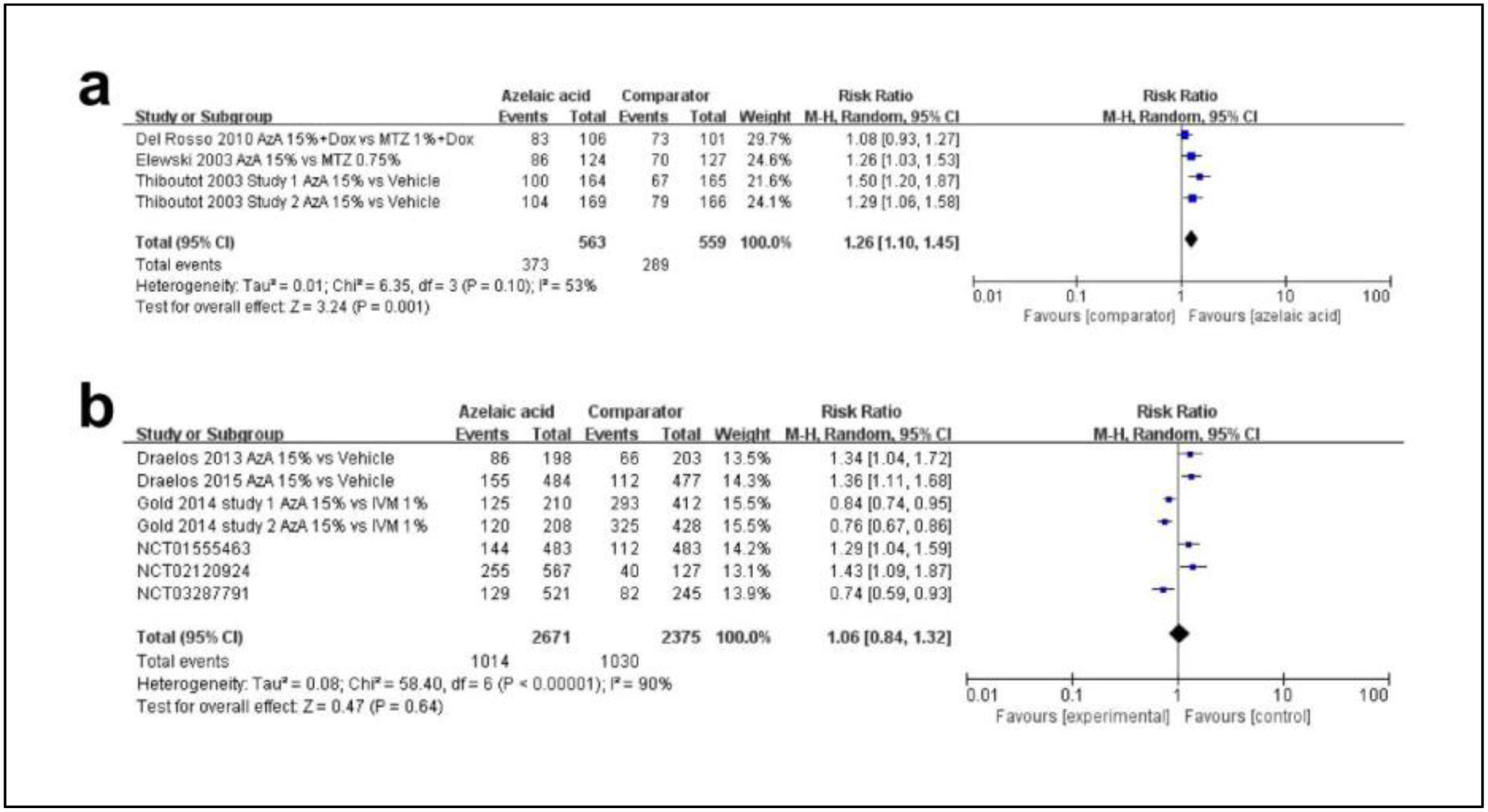
Fifth, regarding the efficacy profile azelaic acid, a total of 9 studies were included. There were 2 kinds of scoring methods in these articles. A total of 3 articles applied a 7-point static scoring system as Table 2 mentioned from 0 (clear) up to 6 (severe). In this system, “success” was defined as IGA≤2 (clear, minimal and mild). As shown in Fig. 4a, the rate of success was higher in the azelaic acid 15% group (MD, 1.26; 95%CI, 1.10–1.45; p=0.001). A total of 6 studies used IGA as Table 3 mentioned. As shown in Fig. 4b, there was no statistically significant difference between azelaic acid and the comparator (MD, 1.06; 95%CI, 0.84–1.32; p=0.64).
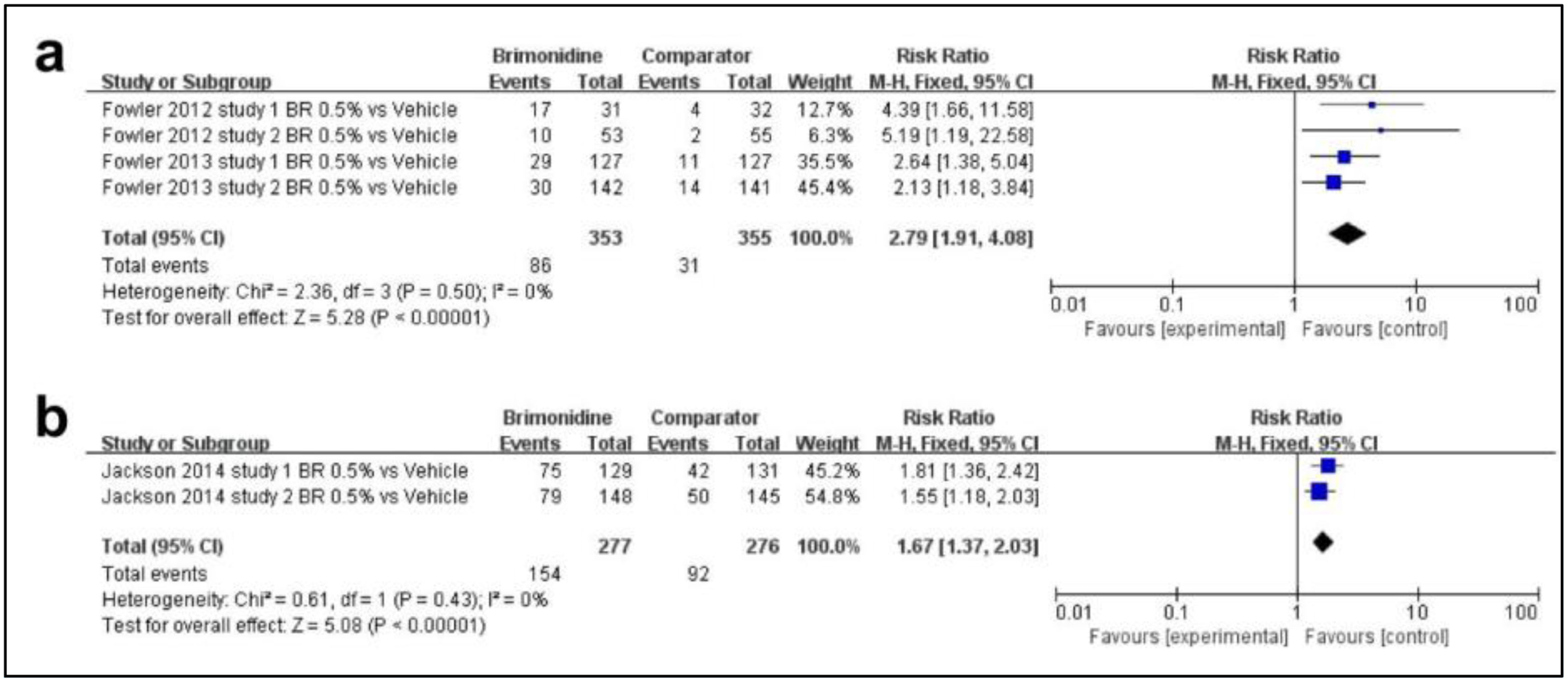
Sixth, regarding the efficacy profile of brimonidine, as shown in Fig. 5a, a total of 2 studies were included. The rate of patients achieving a 2-grade or greater decrease from baseline on both the CEA and the PSA was higher in the brimonidine group and there was significance between the 2 groups (MD, 2.79; 95%CI, 1.91–4.08; p<0.00001). Fig. 5b illustrates 1 article on the rate of patients achieving a 1-grade improvement on both the CEA and the PSA as efficacy outcome. There was also significance between the 2 groups (MD, 1.67; 95%CI, 1.37–2.03; p<0.00001).
DiscussionRosacea is an inflammatory skin disease characterized by immune dysfunction and a neurovascular disorder. Although physicians can alleviate the patients’ symptoms by choosing different potential interventions, it is difficult to cure rosacea.27
When screening related RCTs, various efficacy endpoints were reported. In our study, we used IGA, IGE, CEA, PSA and SSA to quantify the efficacy profiles. IGA was based on the severity of inflammatory lesions (papules and pustules), erythema, and the scoring criteria of IGE was the same as that of IGA. The CEA and PSA were the erythema scoring systems of clinicians and patients, respectively. The SSA was similar to the PSA because they are both based on the patients’ feelings. The 4 scales were relatively simple and clear so we decided to take them as the measurement of outcome indices. Although many related RCTs focused on the change of inflammatory lesion counts, there were no unified data results available for analysis. Since some studies used different erythema grading standards, we decided to excluded them.
Among the drugs studied in this article, minocycline, ivermectin and metronidazole are antibiotics. Minocycline is a broad-spectrum, semi-synthetic second-generation tetracycline which has been demonstrated to have antibacterial and anti-inflammatory properties.28 Minocycline used to be a systematic treatment for rosacea but oral therapy may lead to general side effects such as GI side effects.29 The topical use of it is relatively new. However, it has been reported that the topical application of minocycline provides higher drug concentration and durability in skin layers vs oral administration.30 Minocycline can effectively eliminate external pathogens that cause superficial infections, especially those caused by Gram-positive bacteria.31,32 In the 3 studies included,12–14 minocycline is safe and well-tolerated in patients with papulopustular rosacea.
Metronidazole has been used to treat rosacea for many years and its safety profile has been documented.33 Narayanan S et al. drew the following conclusion from an experiment of skin lipid models: metronidazole exerts antioxidative effect through 2 different ways: by reducing the production of reactive oxygen species (ROS) in tissues and inactivating existing ROS.34 This is probably the main reason behind the clinical efficacy of metronidazole. Topical metronidazole is used to treat rosacea-related inflammatory lesions. Compared to vehicle, metronidazole has a better therapeutic effect on rosacea, yet its efficacy profile is inferior to ivermectin and azelaic acid according to results. Former studies have also demonstrated that metronidazole is effective reducing erythema, papules and pustules.35–39
As for ivermectin, it is an avermectin-class drug which exerts anti-inflammatory effects via inhibition of the production of inflammatory cytokines and upregulation of the anti-inflammatory cytokine IL-10.6,40 It also has been reported that ivermectin exerts anti-parasitic effect.41 In 2020, a study was published on the efficacy profile of ivermectin, whose results were the same as ours.42 No new randomized controlled trials have come out over the past 2 years with results to evaluate the efficacy profile of topical ivermectin. Ivermectin is well-tolerated among patients in the studies included and seems to be more effective than metronidazole and azelaic acid. Besides, a long-term 52-week regimen of ivermectin proved to be safe and effective.11 However, ivermectin has only been used in moderate-to-severe papulopustular rosacea and mainly in Caucasian participants in clinical trials, which limits the universality of the data.43
The pharmacologic mechanisms of azelaic acid have been investigated in many studies, such as the inhibition of microbial survival and viability, regulation of epidermal differentiation and inhibitory action on the generation or release of ROS in neutrophils.44–46 The efficacy profile of azelaic acid in treating rosacea may be due to the inhibition of cathelicidin and kallikrein 5, which are factors considered to play pivotal roles in the pathophysiology of rosacea.47 In our meta-analysis, azelaic acid proved to have a significant effect vs excipients. Furthermore, azelaic acid is always well-tolerated and serves as a feasible treatment option for rosacea patients.
Topical α-adrenergic receptor agonists have been recognized as a treatment for rosacea with persistent facial erythema.24,48,49 Brimonidine has high α2-adrenoceptor affinity and oxymetazoline is a selective α1-adrenergic receptor agonist. These 2 agents bind to the specific receptors on the smooth muscles surrounding the vessels leading to vasoconstriction.48,50 Therefore, these 2 drugs are amenable to treat facial erythema. In the results of our analysis, brimonidine and oxymetazoline proved more effective than the vehicle. The combined use of brimonidine plus ivermectin also increases the success rate of treatment.9 Since the number of RCTs on brimonidine and oxymetazoline is insufficient, we expect more research on the efficacy profile of the 2 drugs.
Although our meta-analysis gave a general overview of topical drugs for rosacea, it still had some limitations. First, most studies included were conducted in America so there was a lack of experimental data among other populations especially in Asia. Differences in the prevalence and severity of the disease among populations from different regions may alter the results of the analysis. Second, the number of studies included on several drugs was limited. Larger-scale clinical trials would be more convincing. Third, since most studies tested topical drugs in patients with moderate (IGA=3) to severe (IGA=4) rosacea, we could not assess the efficacy profile of mild patients (IGA=2). RCTs with the improvement of erythema as an outcome indicator also included participants with moderate-to-severe erythema. More studies conducted with mild patients are still needed. Fourth, there was a lack of comparison between the efficacy profile of multiple drugs although there were more comparison trials across different drugs and vehicles. Therefore, further prospective studies and high-quality studies are required to verify the efficacy profile of multiple topical drugs for rosacea.
ConclusionsThis meta-analysis analyzed the efficacy profile of 6 topical drugs for the treatment of rosacea including minocycline, ivermectin, azelaic acid, metronidazole, brimonidine and oxymetazoline. The efficacy profile of these drugs proved superior to that of vehicles. All these drugs are well-tolerated and safe. Among them, ivermectin proved to be more effective than azelaic acid and metronidazole. Azelaic acid has a better efficacy profile than metronidazole according to included studies. Minocycline proved effective improving the symptoms of rosacea. Brimonidine and oxymetazoline both had a significant effect reducing facial redness. There is also a certain prospect of drug combination application. Studies with larger scale and longer duration will be expected in the future.
FundingThis study was supported by Hangzhou health science and technology key project (No. 20220054). This work was supported by Hangzhou medical key discipline construction project (No. [37]21-3) and Hangzhou biomedical and health industry development support project (2021WJCY159).
Authors’ contributionsXingyue Gao: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Wenzhong Xiang: Writing – review & editing, Funding acquisition. All authors read and approved the final version of the manuscript.
Ethical approvalThis article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Conflicts of interestNone.