Recent studies have shown a relationship between hidradenitis suppurativa (HS) and metabolic syndrome (MS), but the literature offers no meta-analysis restricted to studies that have been adjusted for confounders.
ObjectiveTo determine the association between HS and MS.
MethodsA systematic review and meta-analysis of observational studies on HS and MS in adults. We searched MEDLINE, SCOPUS, SCIELO, Google Scholar, Science Direct and LILACS from the inception of the databases to January 2016. We performed a random effects model meta-analysis for studies reporting adjusted and crude odds ratios (ORs) with 95% CIs. A subgroup analysis was related to the type of HS patient (general patients vs hospital patients) and age group (adults vs children and adults).
ResultsFive studies including 3950 HS patients were analyzed. We found that MS was present in 9.64% of HS patients (OR, 1.82; 95% CI, 1.39–2.25). Studies from tertiary care hospital dermatology clinics (OR, 2.82; 95% CI, 0.58–5.06) reported a greater risk for MS than studies carried out in patients treated outside hospitals (OR, 1.78; 95% CI, 1.34–2.22). Studies that included pediatric populations reported a significant association (OR, 2.10; 95% CI, 1.58–2.62).
LimitationFew of the included studies reported adjusted ORs.
ConclusionsHS patients have an increased risk for MS. Clinicians should consider screening HS patients for metabolic risk factors.
En recientes estudios se ha demostrado la existencia de una relación entre la hidradenitis supurativa (HS) y el síndrome metabólico (SM), sin embargo, hasta el momento no se ha realizado ningún metaanálisis que incluya estudios ajustados según variables de confusión.
ObjetivosDeterminar la asociación entre la HS y el SM.
MetodologíaSe realizó una revisión sistemática y un metaanálisis, donde se incluyeron estudios observacionales acerca de la HS y el SM en pacientes adultos, localizados en MEDLINE, SCOPUS, SCIELO, GOOGLE SCHOLAR, SCIENCE DIRECT y LILACS, y que fueron publicados en un periodo comprendido entre el inicio de la base de datos hasta enero del 2016. De esta manera, se realizó un metaanálisis, para el cual se siguió el modelo de efectos aleatorios basándose en aquellos estudios con odds ratios (OR) ajustadas y crudas y que además presentaban un intervalo de confianza (IC) del 95%. El subgrupo analizado se correlacionó posteriormente con el tipo de población con HS (población general con HS vs. población hospitalaria con HS) y con el grupo de edad (adultos vs. niños y adultos).
ResultadosCinco estudios, incluyendo 3.950 pacientes con HS fueron analizados. Se encontró que el SM estaba presente en el 9,64% de los pacientes con HS (OR 1,82, IC del 95%, 1,39-2,25). Estudios realizados en centros dermatológicos hospitalarios de tercer nivel (OR 2,82, IC del 95%, 0,58-5,06) mostraron una mayor asociación al SM si comparados con estudios realizados en la población general (OR 1,78, IC del 95%, 1,34-2,22). Los estudios que incluyeron una población pediátrica registraron así mismo una asociación significativa al SM (OR 2,10, IC del 95%, 1,58-2,62).
LimitaciónSe contó con escasos estudios ajustados a las variables de confusión.
ConclusiónLos pacientes con HS tendrán un riesgo incrementado de presentar SM. Se debería considerar la realización de un cribado de factores de riesgo metabólicos en todos los pacientes con HS.
Hidradenitis suppurativa (HS) is a chronic, recurring suppurative skin disease that affects the apocrine glands, usually in the armpits and groin, and causes painful, deep abscesses and sinus tracts.1,2
Metabolic syndrome (MS) encompasses a combination of four metabolic alterations: obesity, diabetes mellitus, dyslipidemia, and arterial hypertension. When all the components are present simultaneously, the risk of cardiovascular disease increases, shortening life expectancy.3,4
Sabat et al5 were the first group to demonstrate an association between HS and MS. The association they found between chronic inflammation in HS and metabolic changes was similar to the association between psoriasis and MS, as recently analyzed.6 An earlier meta-analysis showed that patients with HS have a 2-fold higher risk for MS (cumulative odds ratio [OR], 2.22; 95% CI, 1.62–3.06), although the studies included at that time had not reported ORs adjusted for the influence of possible confounders and the meta-analysis had not considered heterogeneity and biases.8
These findings have important implications for therapy over the chronic, relapsing course of the disease, considering that standard approaches to treating HS are still suboptimal. Alternative therapies may prove appropriate in HS; for example, one study found that metformin helped control HS symptoms with few adverse effects.9
We aimed to review the current literature to compare reported prevalence rates of MS in HS and assess the association between the 2 conditions. We sought studies that had adjusted for possible confounders.
MethodsThis systematic review and meta-analysis followed the MOOSE guidelines (Meta-analysis of Observational Studies in Epidemiology).10 The protocol was registered in the PROSPERO system (CRD42017059937).
Inclusion CriteriaWe included observational studies with case–control and cross-sectional designs that described the association of HS and MS. The studies could be either prospective or retrospective, and the language of publication could be either English or Spanish. Searches encompassed the time from inception of the databases until January 2016.
Study participants could be either children or adults with HS. Control patients did not have HS. Both sexes were included. The studies had to assess the prevalence or incidence of MS, as defined by any of the following groups: the World Health Organization,11 the National Cholesterol Education Program Adult Treatment Panel III (ATP-III),12 the International Diabetes Federation,13 and the Joint Scientific Statement (of the American Heart Association among others).14 Each definition includes several clinical criteria based on physical examination as well as laboratory findings or patient-reported information in both the HS and control populations.
After making a concerted attempt to contact the authors of articles with missing information, we discarded any studies that still lacked crude or adjusted OR data. If studies included overlapping data, only the one with the larger number of cases was retained.
Sources and Search StrategySearches were done in the following databases: MEDLINE, SCOPUS, SCIELO, Google Scholar, Science Direct, and LILACS. A search strategy appropriate for each database was used. We used Medical Subject Headings and the following free-text terms hidradenitis suppurativa (Spanish, hidradenitis supurativa) and metabolic syndrome (síndrome metabólico). (See online supplementary material, Appendix.) We also hand searched journals for relevant articles mentioned in reference lists.
Study Selection and Data ExtractionTwo researchers (M.J.M.R.-Z. and H.A.G.-P.) independently read titles and abstracts to assess each study's relevance. The selection criteria were then applied to full texts before articles were finally included. Any disagreement between the readers was resolved by consulting the third author (A.G.O.-L.). The 2 readers then extracted data independently, filling in a standardized questionnaire. Both checked all entries and revised the data sheets at least twice to ensure completeness and accuracy. The following information was extracted: author, year of publication, country/geographic location, study design, number and type of patient; mean age, prevalence or incidence of MS in HS and control groups; and crude and adjusted ORs.
Definitions of VariablesAll the included studies applied the ATP-III criteria to define MS.12 Thus, MS was diagnosed when a patient had at least 3 of the following conditions1: fasting glucose levels of 100mg/dL (or under treatment to lower blood sugar levels),2 blood pressure of at least 130/85mmHg (or on antihypertensive treatment),3 triglyceride concentration of 150mg/dL or more (or on treatment),4 high density lipoprotein cholesterol <40mg/dL in men or <50mg/dL in women (or on treatment),5 or a waist circumference over 102cm in men or 88cm in women.
The primary objective was to estimate the association between HS and MS in studies reporting ORs adjusted for confounders such as age, sex, and smoking. Four secondary objectives were also set: 1) to estimate the association between HS and MS based on reported crude ORs, 2) to estimate the association in subgroups, 3) to describe the characteristics of patients with HS (age, sex, smoking habit, etc.), and 4) to assess risk of bias in the included studies.
Data AnalysisWe used adjusted ORs reported in the studies to calculate the cumulative OR. This OR, reported with its 95% CI, was the outcome measure for the primary objective. Because we expected considerable heterogeneity, the cumulative OR was calculated using a random effects meta-analysis, which is the standard approach.15 Statistical heterogeneity was determined by the Q test, which is based on the χ2 test and the I2 statistic,16 which is considered high if over 50%.17
Descriptive data from clinical trials (percentages and means) were analyzed. All analyses and meta-analyses were carried out with Stata13 software (StataCorp). Some images were created with Review Manager, version 5.1 (Cochrane Collaboration).
Subgroup AnalysesWe compared results according to design (cross-sectional vs case–control), data collection methods (prospective vs retrospective), geographic location (Europe vs other), MS diagnostic criteria (ATP-III vs other), and patient type (hospital vs another “general” source and adults vs children). Forest plots were used to report the effect sizes and 95% CIs. These subgroup analyses were based on adjusted ORs whenever possible or on crude ORs if no adjusted ORs were reported.
Publication BiasPublication bias was assessed by inspecting funnel plots of study size against standard error. A symmetrical plot indicates that publication bias is unlikely except when very small studies are included. (See online supplementary material, Appendix.)
Quality AssessmentWe used the MINORS tool (Methodological Index for Non-Randomized Studies) to assess methodological quality.18 Two authors (M.J.M.R.-Z. and A.G.O.-L.) completed their assessments independently. Items were scored 0 if not reported, 1 if reported but inadequately, and 2 if reported adequately. The ideal overall score for a comparative study was 24, and we judged that the risk of bias was low if the score was at least 20. (See online supplementary material, Appendix.) When the 2 independent raters differed on any of the items, they attempted to reach agreement. If they failed, the third author (H.A.G.-P.) was consulted for an independent opinion.
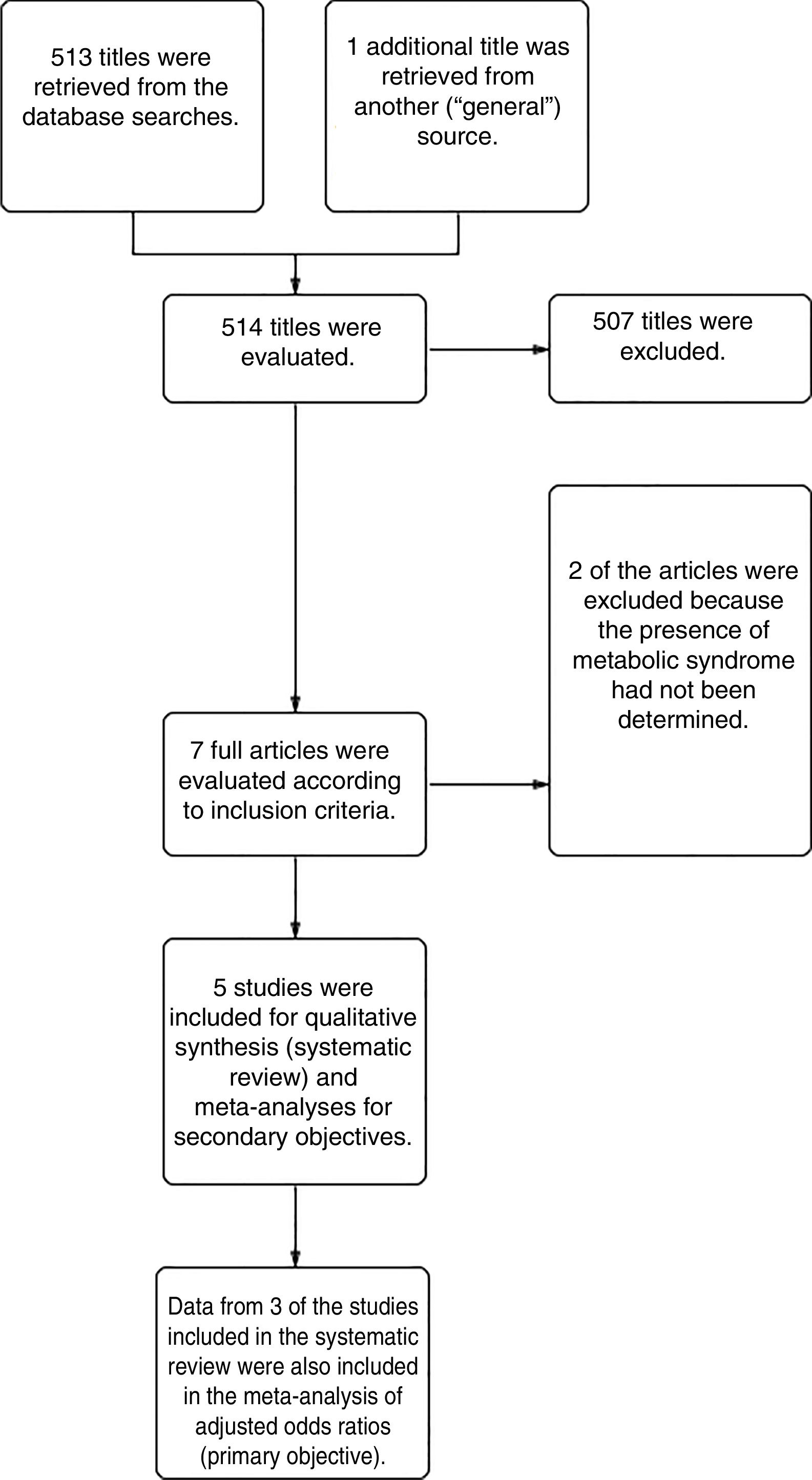
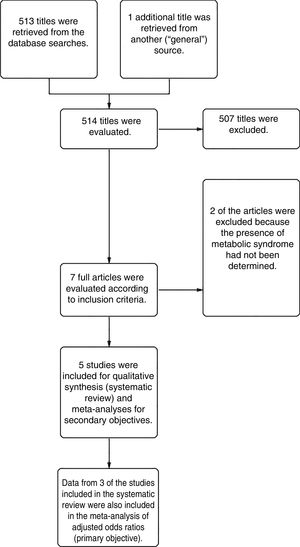
ResultsFrom a total of 514 articles retrieved from searches we selected 5studies after applying the inclusion criteria for this systematic review. Three of the studies adjusted for confounding factors and were included in the meta-analysis to meet the primary objective. All 5studies reported crude ORs and were included in the meta-analysis to meet the secondary objective (Fig. 1).
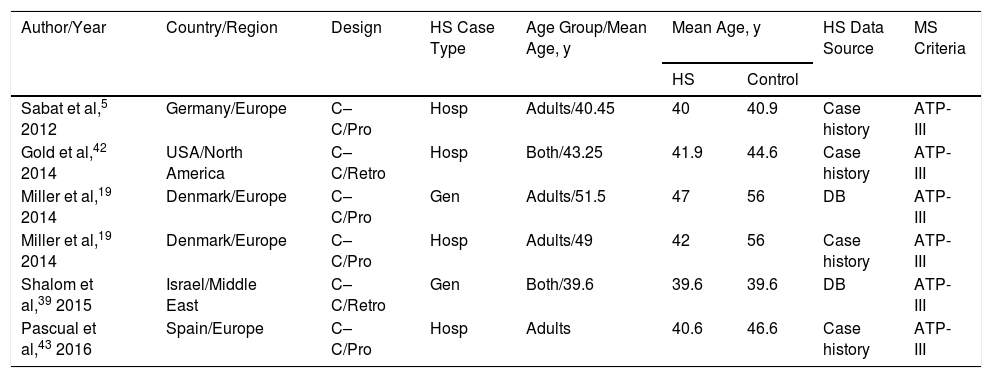
All were retrospective or prospective case–control studies and had been carried out in Europe, North America, or the Middle East. Patients with HS were identified in electronic databases or other hospital records. Included patients were being treated in dermatology clinics located in tertiary referral hospitals or elsewhere (termed “general”). Two patient cohorts were analyzed in the study by Miller et al19: one included hospital patients with HS and the other included persons with HS from general sources (Table 1).
Studies Included in the Systematic Review in Order of Year of Publication
| Author/Year | Country/Region | Design | HS Case Type | Age Group/Mean Age, y | Mean Age, y | HS Data Source | MS Criteria | |
|---|---|---|---|---|---|---|---|---|
| HS | Control | |||||||
| Sabat et al,5 2012 | Germany/Europe | C–C/Pro | Hosp | Adults/40.45 | 40 | 40.9 | Case history | ATP-III |
| Gold et al,42 2014 | USA/North America | C–C/Retro | Hosp | Both/43.25 | 41.9 | 44.6 | Case history | ATP-III |
| Miller et al,19 2014 | Denmark/Europe | C–C/Pro | Gen | Adults/51.5 | 47 | 56 | DB | ATP-III |
| Miller et al,19 2014 | Denmark/Europe | C–C/Pro | Hosp | Adults/49 | 42 | 56 | Case history | ATP-III |
| Shalom et al,39 2015 | Israel/Middle East | C–C/Retro | Gen | Both/39.6 | 39.6 | 39.6 | DB | ATP-III |
| Pascual et al,43 2016 | Spain/Europe | C–C/Pro | Hosp | Adults | 40.6 | 46.6 | Case history | ATP-III |
Abbreviations: Both, pediatric and adult patients; ATP-III, National Cholesterol Education Program Adult Treatment Panel III diagnostic criteria for MS; C–C, case–control; DB, electronic database; HS, hidradenitis suppurative; aOR, adjusted odds ratio; cOR, crude odds ratio; Hosp, hospital HS patients; Gen, “general” population HS cases; Pro, prospective; Retro, retrospective; MS, metabolic syndrome.
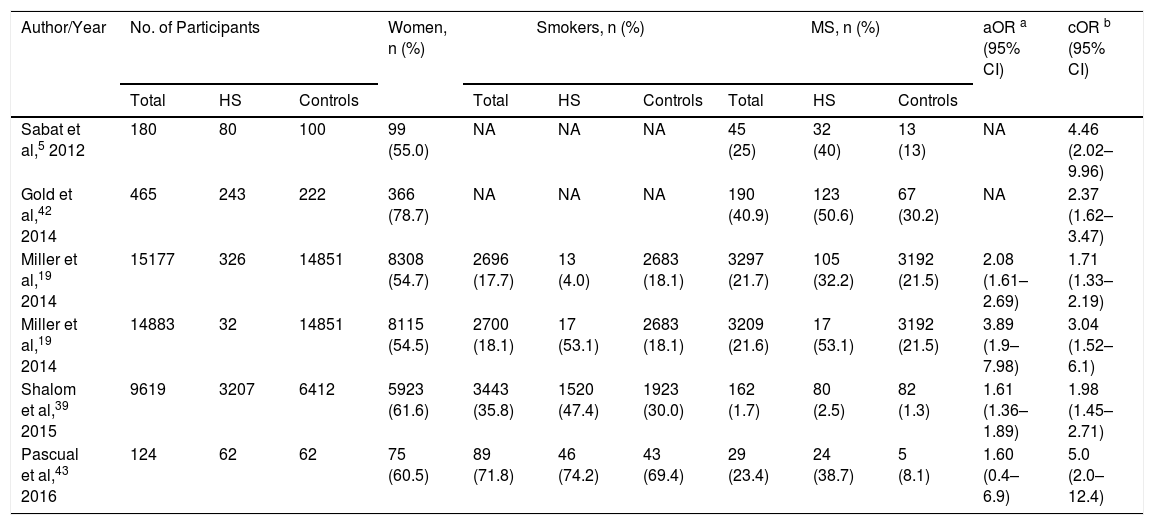
The systematic review encompassed a total of 25597 participants, of whom 3950 with HS had a mean age of 44.5 years. Both sexes and both children and adults were included (Table 2). The overall prevalence of MS was 9.64% among the HS patients.
Meta-Analyzed Data from Included Studies, in Order of Year of Publication.
| Author/Year | No. of Participants | Women, n (%) | Smokers, n (%) | MS, n (%) | aOR a (95% CI) | cOR b (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | HS | Controls | Total | HS | Controls | Total | HS | Controls | ||||
| Sabat et al,5 2012 | 180 | 80 | 100 | 99 (55.0) | NA | NA | NA | 45 (25) | 32 (40) | 13 (13) | NA | 4.46 (2.02–9.96) |
| Gold et al,42 2014 | 465 | 243 | 222 | 366 (78.7) | NA | NA | NA | 190 (40.9) | 123 (50.6) | 67 (30.2) | NA | 2.37 (1.62–3.47) |
| Miller et al,19 2014 | 15177 | 326 | 14851 | 8308 (54.7) | 2696 (17.7) | 13 (4.0) | 2683 (18.1) | 3297 (21.7) | 105 (32.2) | 3192 (21.5) | 2.08 (1.61–2.69) | 1.71 (1.33–2.19) |
| Miller et al,19 2014 | 14883 | 32 | 14851 | 8115 (54.5) | 2700 (18.1) | 17 (53.1) | 2683 (18.1) | 3209 (21.6) | 17 (53.1) | 3192 (21.5) | 3.89 (1.9–7.98) | 3.04 (1.52–6.1) |
| Shalom et al,39 2015 | 9619 | 3207 | 6412 | 5923 (61.6) | 3443 (35.8) | 1520 (47.4) | 1923 (30.0) | 162 (1.7) | 80 (2.5) | 82 (1.3) | 1.61 (1.36–1.89) | 1.98 (1.45–2.71) |
| Pascual et al,43 2016 | 124 | 62 | 62 | 75 (60.5) | 89 (71.8) | 46 (74.2) | 43 (69.4) | 29 (23.4) | 24 (38.7) | 5 (8.1) | 1.60 (0.4–6.9) | 5.0 (2.0–12.4) |
Abbreviations: HS, hidradenitis suppurative; aOR, adjusted odds ratio; cOR, crude odds ratio; MS, metabolic syndrome; NA, not applicable.
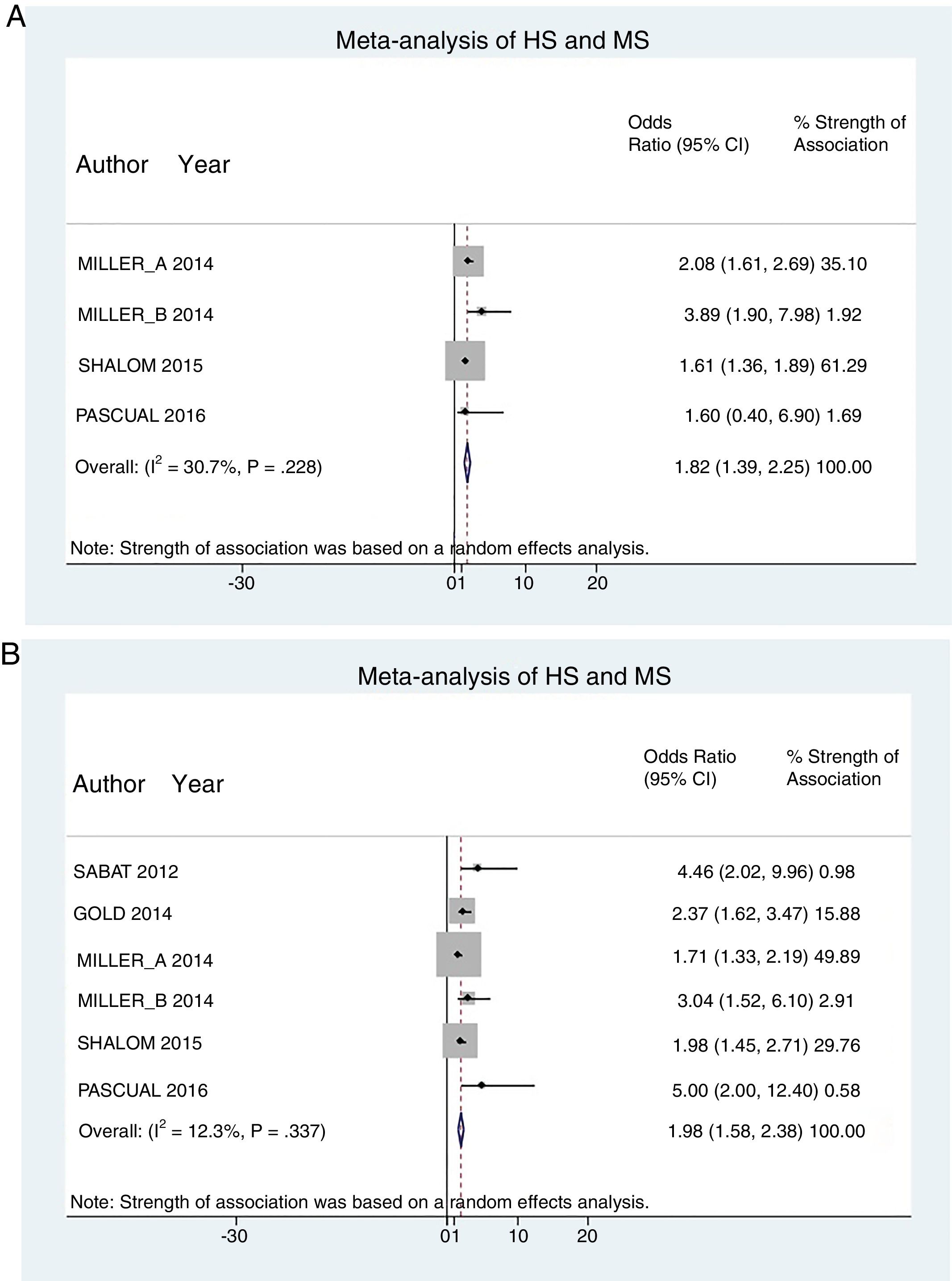
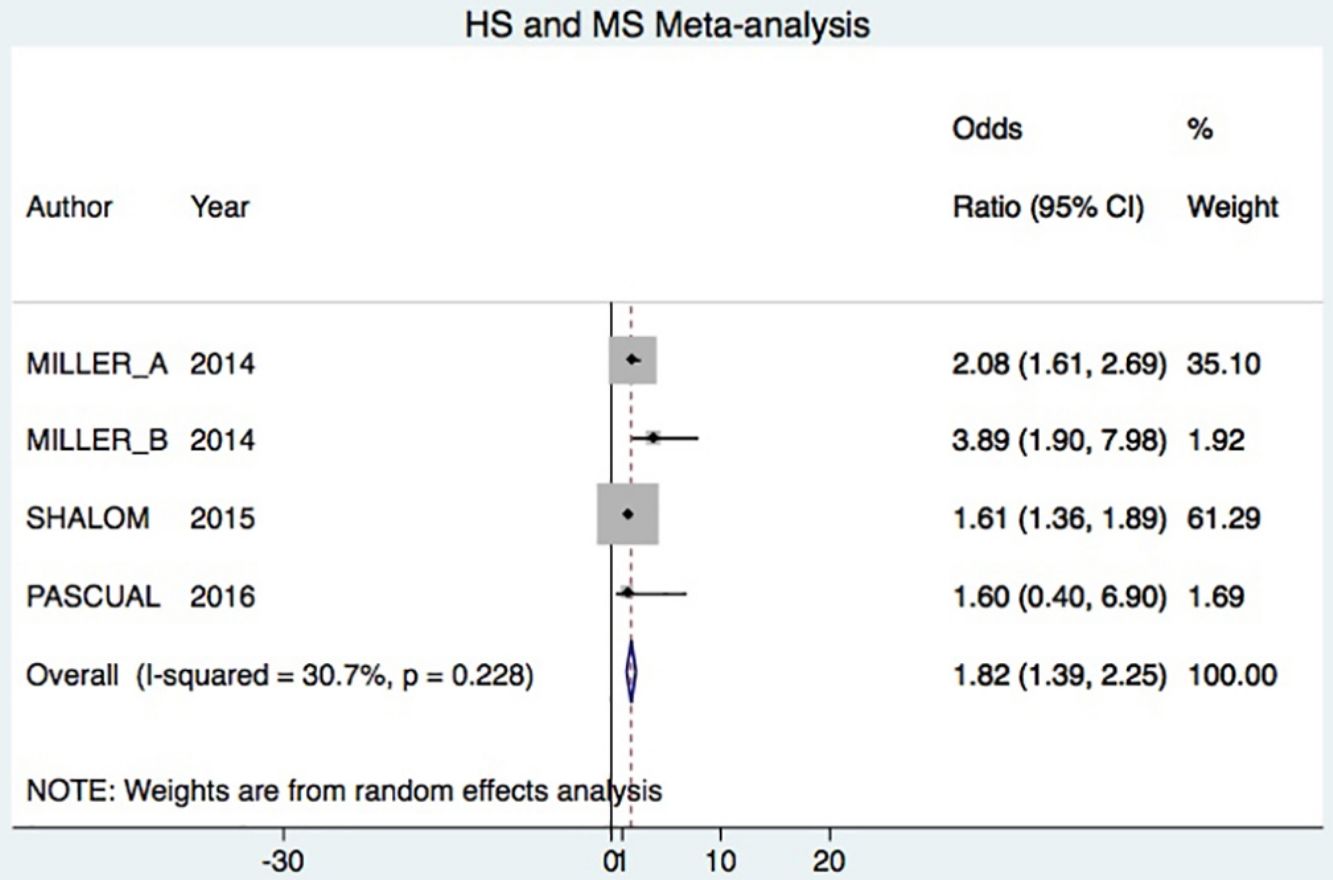
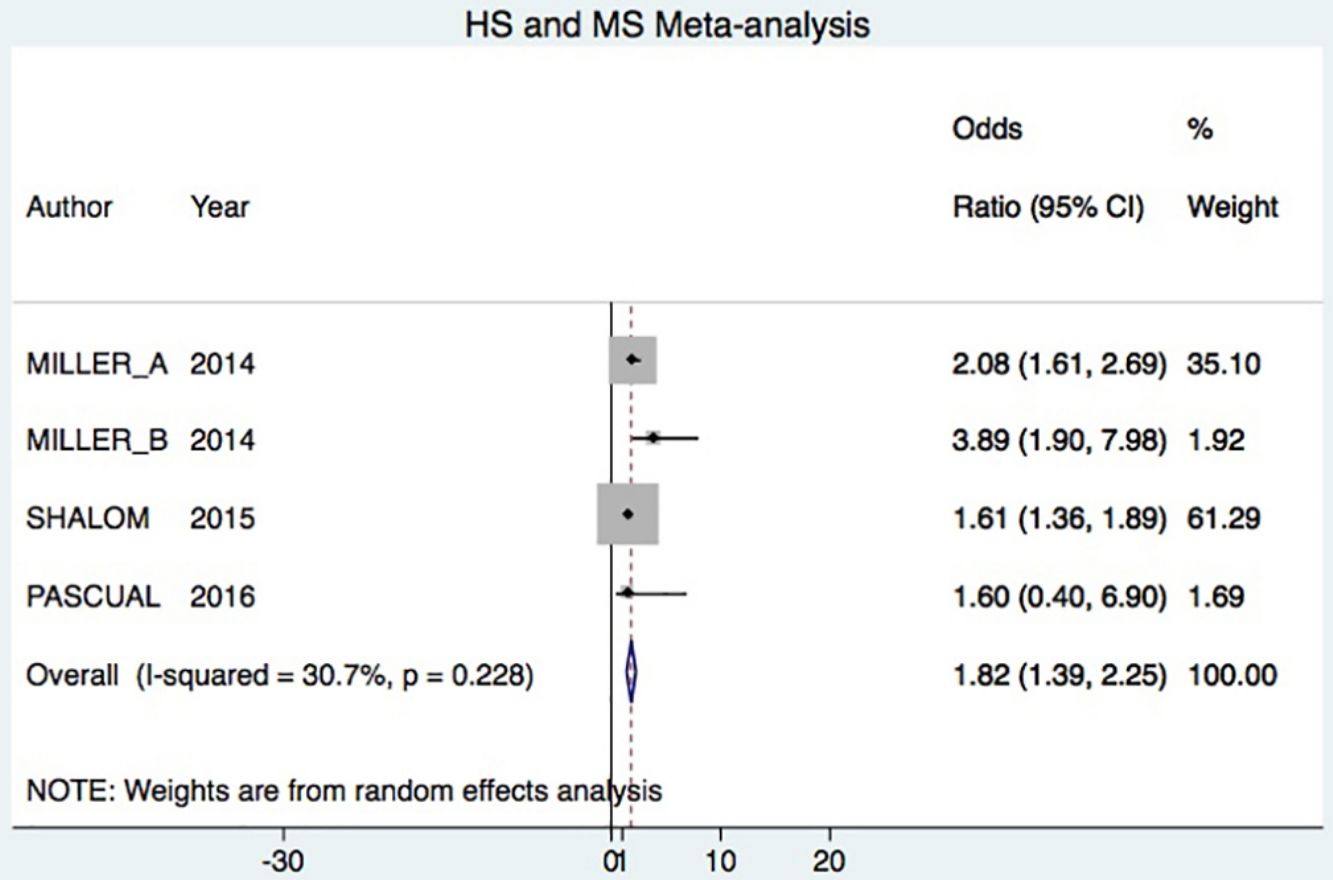
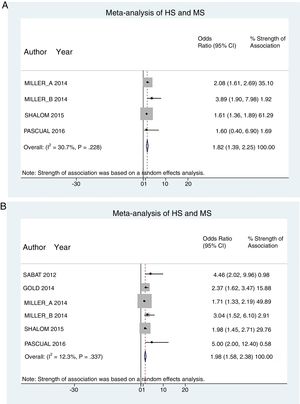
A total of 24952 participants entered the meta-analysis of studies that adjusted for confounding factors (primary objective); 3627 were patients with HS (cases) and 21325 were controls without HS. MS was present in 226 cases and 3279 controls, giving a cumulative OR for MS of 1.82 (95% CI, 1.39–2.25; I2=30%) for the cases (Fig. 2A).
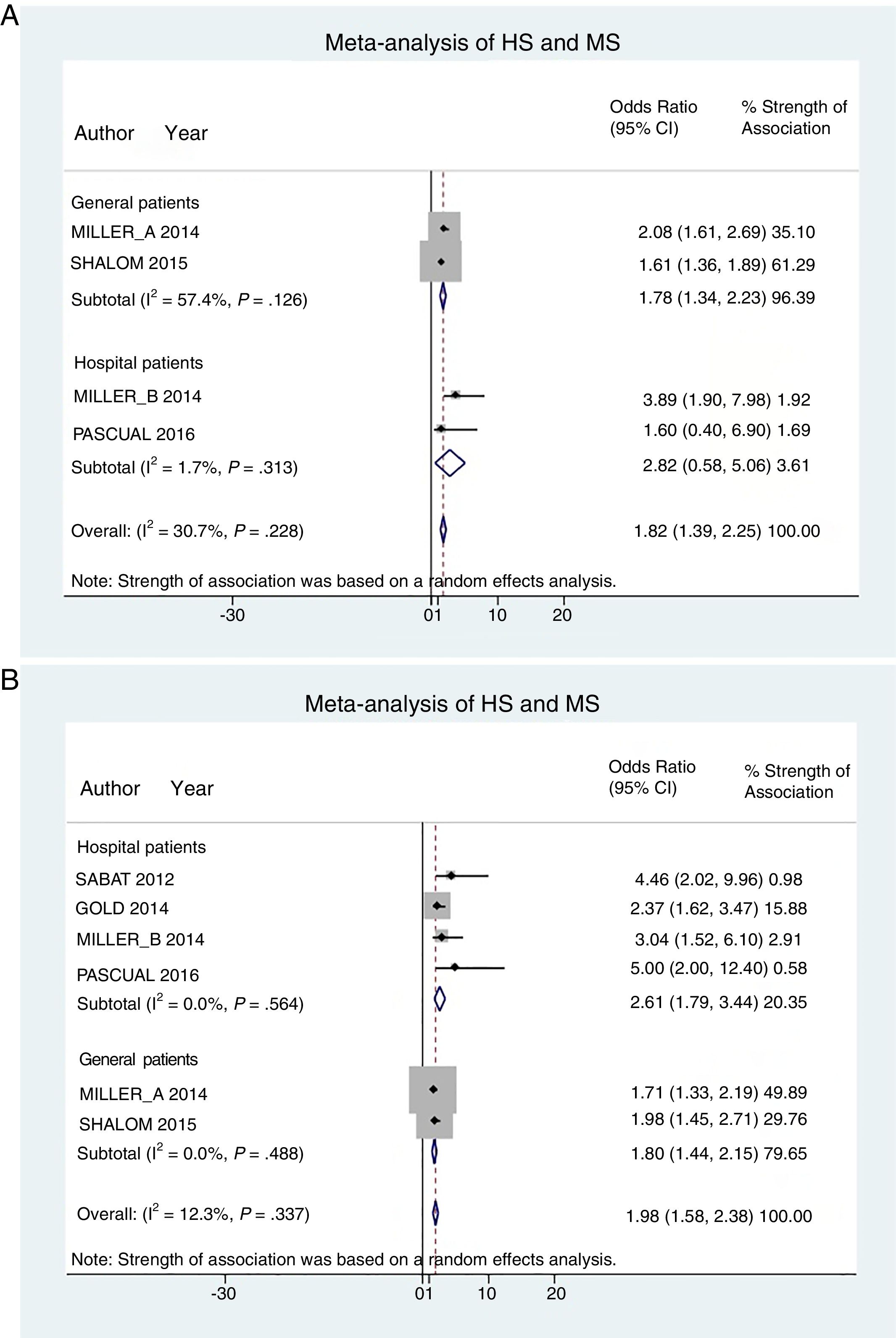
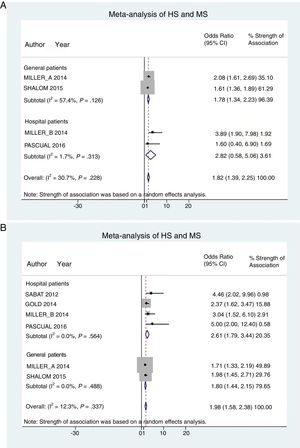
Hospital patients were at higher risk of presenting MS (OR, 2.82; 95% CI, 0.58–5.06; I2 = 2%) than others (OR, 1.78; 95% CI, 1.34–2.22; I2 = 57%) (Fig. 3A).
Studies done in Europe (Denmark, Germany, and Spain) found that patients with HS had significant risk of presenting MS (OR, 2.12; 95% CI, 1.60–2.65; I2 = 0%).
The meta-analysis of studies reporting crude ORs showed that 381 HS patients and 6551 controls had MS, giving a cumulative OR of 1.98 (95% CI, 1.58–2.38; I2 = 12%) (Fig. 2B).
Patient subgroup analyses, using data from studies reporting crude ORs, showed that hospital HS patients had a slightly higher risk of presenting MS (OR, 2.61; 95% CI, 1.79–3.34; I2 = 0%) than other (general) patients (OR, 1.80; 95% CI, 1.44–2.15; I2 = 0%) Fig. 3B).
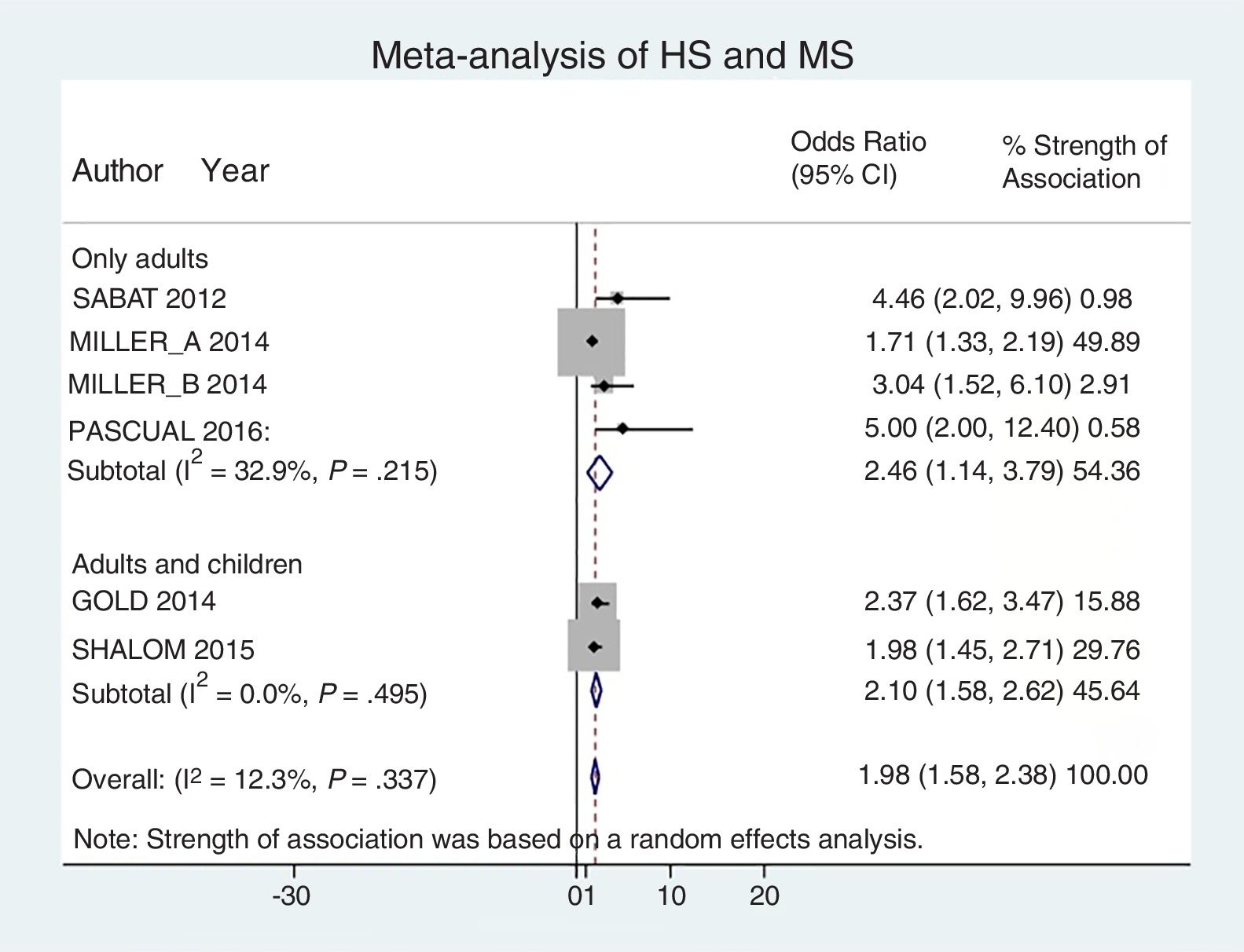
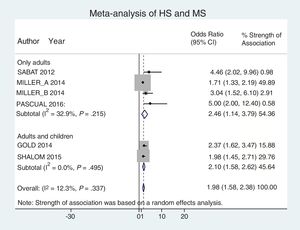
Analysis by age groups using data from studies that included pediatric patients showed that they had a 2-fold higher risk of having MS (OR, 2.10; 95% CI, 1.58–2.62; I2 = 0%). The association was similar in studies that included only adults (OR, 2.46; 95% CI, 1.14–3.79; I2 = 33%) (Fig. 4).
A total of 6156 participants (cases and controls) were smokers: 40% were HS patients and 20% were controls. HS patients who smoked had a 2-fold higher risk of presenting MS than controls (OR, 1.85; 95% CI, 1.70–2.00; I2 = 96%).
When the data was subjected to metaregression analysis of characteristics (age and type of participant), the strength of the association between HS and the presence of MS did not reach significance in studies adjusting for confounding factors (P=0.336).
In addition, the funnel plots (online supplementary material, Appendix) and the Egger test of asymmetry (coefficient of bias=3.74, P=.000) suggested the presence of publication bias.
DiscussionHS–MS Association in Studies With Adjusted vs Crude ORsThe meta-analysis included studies with ORs adjusted for confounding factors as well as crude ORs. The 2 types of data were analyzed separately. The primary objective was met by analyzing only studies reporting adjusted ORs, in keeping with Cochrane recommendations.20 Our analysis showed that HS patients had approximately 80% higher risk of MS compared to control patients. This result was similar to the findings of an earlier meta-analysis, which reported a cumulative OR of 2.22 (95% CI, 1.62–3.06; I2 = 77%).7
Observational studies generally suffer from a higher likelihood of bias from confounding factors. Therefore, and because the observational studies we included had different designs, we preferred adjusted ORs in order to reduce effects of bias and heterogeneity.8
However, we did analyze crude ORs for our secondary objectives, following a method similar to that of an earlier meta-analysis.7 Our results with crude ORs similarly showed that HS patients had a 2-fold higher risk for MS. It is believed that the similarity between adjusted and crude ORs is attributable to the small number of published studies. However, the similarity does suggest there is an association between HS and MS.
The HS–MS association is related to a shared origin in chronic inflammation. As in psoriasis,21–23 chronic inflammation in HS leads to insulin resistance and, subsequently, endothelial cell dysfunction, promoting cardiovascular disease. Although there is no consensus about which cytokines and immune pathways are responsible for inflammation in HS, studies have demonstrated the presence of increased concentrations of interleukin (IL) 17, IL-1b, IL-10, tumor necrosis factor, and IL-23, which are considered the main cytokines involved.24,25
HS–MS Association in Tertiary Care Hospital Dermatology Clinics vs General CasesWe also found a stronger association between HS and MS in hospital-treated patients versus “general” patients. These findings reflect diagnostic bias between these 2 groups. However, we believe that tertiary care hospital clinics are more likely to be treating HS patients with more severe symptoms, such as a larger number of lesions or highly inflamed lesions, which would have a greater impact on quality of life.
This indirect indicator of disease severity is reflected in studies reporting that patients with more severe forms of HS seek care in dermatology clinics, increasing the burden on the health care system.26,27 Hence, we believe this pattern explains the stronger HS–MS association in groups with more severe disease7,28 mediated by a higher degree of inflammation in severe HS.29 Hessametal30 found that HS severity correlated positively with higher concentrations of serum markers of inflammation such as C-reactive protein and neutrophils.
Each component of MS (arterial hypertension, obesity, dyslipidemia, and diabetes mellitus) will also show an association with HS. A higher body mass index has been linked to greater severity in HS symptoms,31,32 and a significant correlation between HS and obesity in contrast with overweight has been reported (OR, 4.4 vs 2.1).28 The prevalence of obesity in patients with HS (70%) was even higher than in patients with psoriasis (50%) in one study.33
HS–MS Association in Smokers and NonsmokersPatients with HS were 2-fold more likely than control patients to be smokers. There is considerable epidemiologic evidence for a link between HS and smoking,32 and it is important to remember that smoking will contribute considerably to inflammation in MS, exacerbating and accelerating atherogenesis.34 Nonsmoking patients with HS have also been reported to improve with treatment more than smokers.35 Our findings support the notion that smoking addiction is a common concomitant condition among patients with HS. Thus, physicians should encourage these patients to quit smoking in order to improve both their HS symptoms and their metabolic profile.
HS–MS Association in Children and AdultsHS symptoms, which usually appear after puberty and are less common during childhood,36 can nevertheless develop at young ages.37 Our analysis shows that there is a significant association between HS and MS in studies that include pediatric patients as well as adults. However, there is scarce literature on the relationship between obesity and MS in children with HS.
Deckers et al38 found that the body mass indexes of patients whose HS symptoms developed after the age of 30 years were similar to those whose symptoms appeared earlier. When Shalometal39 analyzed their results by age groups in a study comparing age- and sex-matched patients with HS, the prevalence of obesity was significantly higher in HS patients under the age of 20 years (20%) than in older patients (12%) (P=.005).
Finally, current guidelines state that the possibility of endocrine disorders and obesity should be borne in mind when treating young patients with HS.40 MS, which is a condition that includes diabetes mellitus, hypertension, and dyslipidemia among its components, does not have specific criteria for making the diagnosis in children.41 Studies specifically in pediatric cohorts should be carried out to confirm the association between HS and pediatric metabolic disorders.
LimitationsThe funnel plots we constructed suggest the presence of publication bias. (See online supplementary material, Appendix.) This detection of probable bias contrasts with an earlier meta-analysis.7 However, heterogeneity among the studies we included was low, as reflected in the consistency of their findings. Likewise, the risk of bias was low in a considerable number of the individual studies (Figs. 2A and 2B), supporting the validity of our findings. These results should nevertheless be interpreted cautiously.
In addition, we found few studies about HS and MS and were unable to obtain missing data from authors in spite of repeated attempts to reach them. Finally, potentially eligible studies may have been left out because we restricted our search to English and Spanish, leaving out such languages as German and Chinese.
ConclusionThis meta-analysis detected an association between HS and MS in both adult and pediatric patients. We also saw a relation between MS and severity of HS. These findings underline the need to screen HS patients for MS. Smoking should also be discouraged in HS. We suggest carrying out more studies to assess the clinical significance of the HS–MS association in order to investigate the possible efficacy and safety of medications used in MS as treatments for HS. Trials should be designed to determine whether these treatments might have an effect on the metabolic profile of these patients as well as improve their HS symptoms.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rodríguez-Zuñiga MJM, García-Perdomo HA, Ortega-Loayza AG. Asociación entre la hidradenitis supurativa y el síndrome metabólico: revisión sistemática y metaanálisis. Actas Dermosifiliogr. 2019;110:279–288.