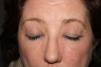
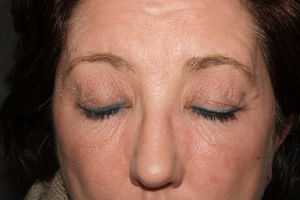
A 54-year-old woman presented at our dermatology unit with facial erythema associated with itching and a burning sensation. She had been applying Neoretin Serum (Cantabria Lab., Santander, Spain) to her face for approximately 1 month (Fig. 1). She was instructed to stop using this product and was prescribed topical hydrocortisone until the erythema improved.
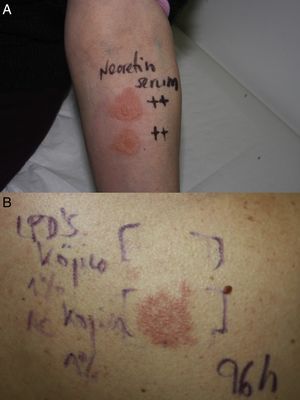
In accordance with the European Society of Contact Dermatitis guideline for diagnostic patch testing,1 the patient was tested with the standard series recommended by the Spanish Contact Dermatitis Research Group (GEIDAC), supplied by BIAL-Aristegui (Bilbao, Spain), and Nerotetin Serum (semi-occlusive patch test) on her forearm. The 48- and 96-hour readings were positive (+++) for the serum only (Fig. 2A).
Four weeks later, the patient was tested with the individual ingredients of the serum (with petrolatum as the vehicle) kindly supplied by the manufacturer (Cantabria Labs, SL). These included hydroxypinacolone retinoate, N-acetylglucosamine, Cromabright, Natriquest, Alistin, Albatín, niacinamide, HidraCare, Hydromanil, acetyl hexapeptide, Physalis angulata extract, Portulaca oleracea extract, salicylic acid, kojic acid nanoliposomes, and kojic acid. The substances were tested as supplied by the manufacturer, except for kojic acid, which was applied in a 1% water solution, as previously described.2 The patient showed no reaction at 48hours, but re-evaluation at 96hours showed a strong positive reaction (++) to kojic acid (Fig. 2B). The reaction was still present at day 7. The rest of the ingredients were negative.
The kojic acid 1% solution was tested in 12 control patients and was negative in all cases at 96hours.
Kojic acid (5-hydroxy-2-(hydroxymethyl)-4-pyrone) is widely used as a cosmetic skin lightening product and its depigmentation properties are due to its ability to chelate copper from free tyrosinase.
Kojic acid is a natural substance produced by fungi and bacteria such as Aspergillus, Penicillium and Acetobacter spp.2 It is also a traditional Japanese ingredient found in miso (soy pasta), shoyu (soy sauce), and sake.2 There is no evidence that the doses present in food are harmful,3 or that eating food containing kojic acid causes dermatitis recurrence or any other adverse effects in patients with contact dermatitis to kojic acid.2
Although kojic acid is widely used, few cases of contact dermatitis to this substance have been published.2,4,5 There have also been reports of leucoderma (hypopigmentation)6 and even paradoxical hyperpigmentation following its use.7
In the largest series to date of contact dermatitis due to kojic acid, Nakagawa and Kawai2 found that sensitization occurred within a relatively short period (within 1-12 months of use), probably due to the frequent application of the product and the strong patch test reactions, particularly on days 3 and 7, as in our case.
The authors remarked on the intensity of the patch test reactions, with stronger reactions seen on days 3 and 7 than at 48hours. We observed the same in our patient.
We have described a case of contact dermatitis to topical kojic acid. Although there are very few reports in the literature, it is important to be familiar with this condition, as kojic acid is widely used in skin lightening products.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Tejera-Vaquerizo A, García-Gavín J. Dermatitis alérgica de contacto al ácido kójico. Actas Dermosifiliogr. 2019;110:243–244.