Paraneoplastic autoimmune syndromes related to thymoma like myasthenia gravis, lupus erythematosus, hypogammaglobulinemia and cytopenia are well-known. Less frequently, this immune dysregulation can lead to different skin manifestations such as bullous diseases, lichen planus, alopecia or TAMA (“thymoma-associated multiorgan autoimmunity”).1–4
A 46-year-old male diagnosed with bone narrow aplasia associated with an unresectable advanced stage thymoma (Type B1/B2, Masaoka stage IVa) was referred to our department. He received multiple lines of chemotherapy (discontinued 3 moths ago) with stabilization of the tumour and he was treated with cyclosporine for 1 year because of the aplasia, which appeared prior to systemic treatments and linked to the autoinmune process triggered by the thymoma.
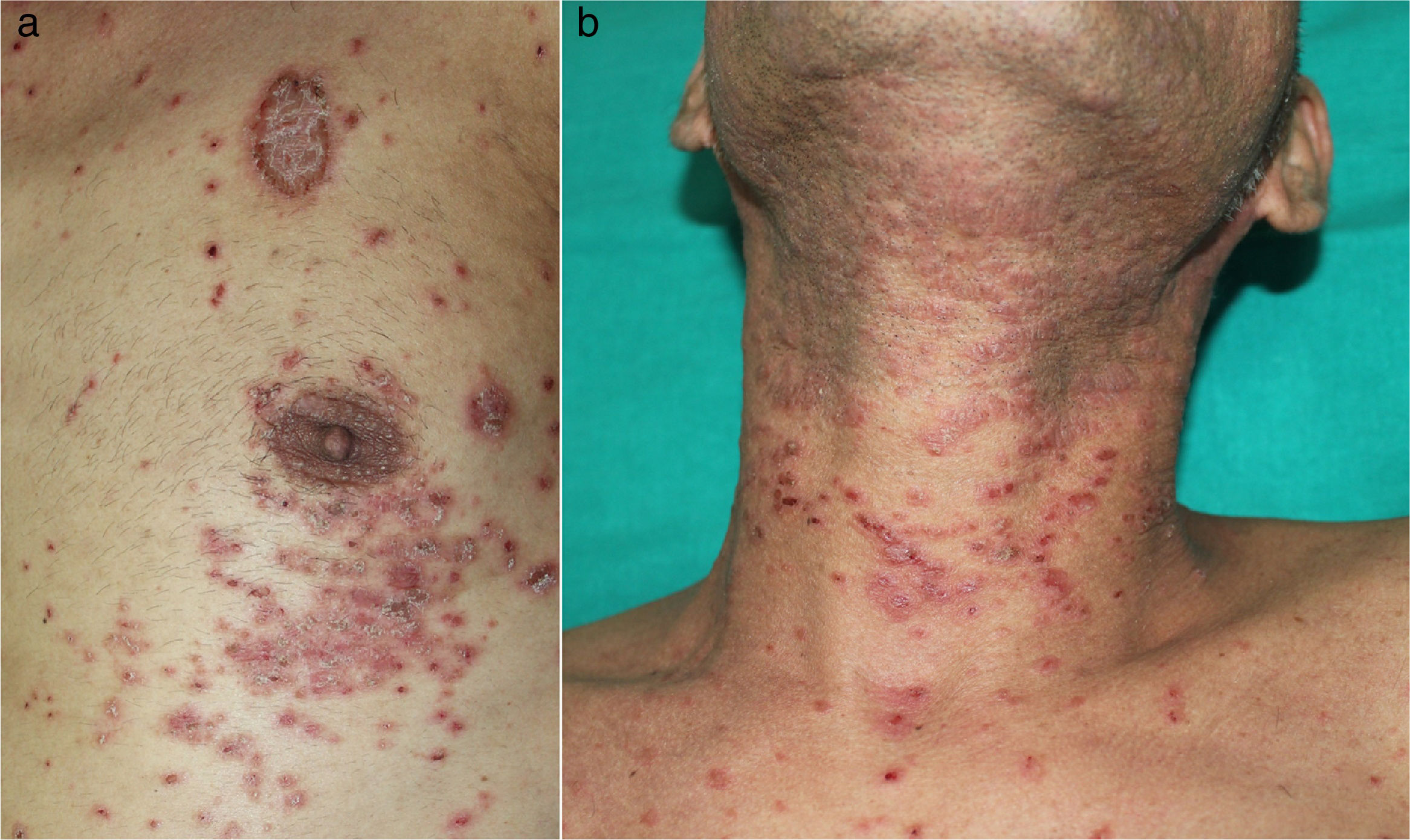
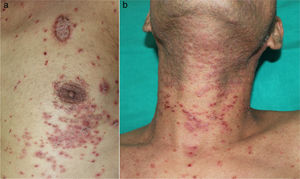
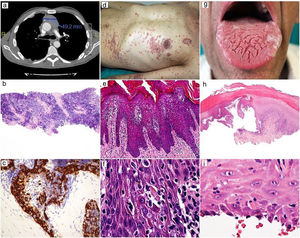
He had a two-week history of pruritic skin rash showing multiple papular keratotic lesions located on the extremities and trunk (Fig. 1 a, b). He also had a marked periorbital and peribuccal hyperpigmented lesions, dryness and scaling of the lips and painful eroded whitish reticulated lesions involving the tongue and oral mucosa. Progressive dysphagia and persistent diarrhoea were also present. No blood transfusions or solid-organ transplantation were performed before the onset of the lesions. (Fig. 2 a, d, g).
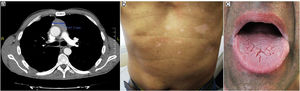
Before treatment.
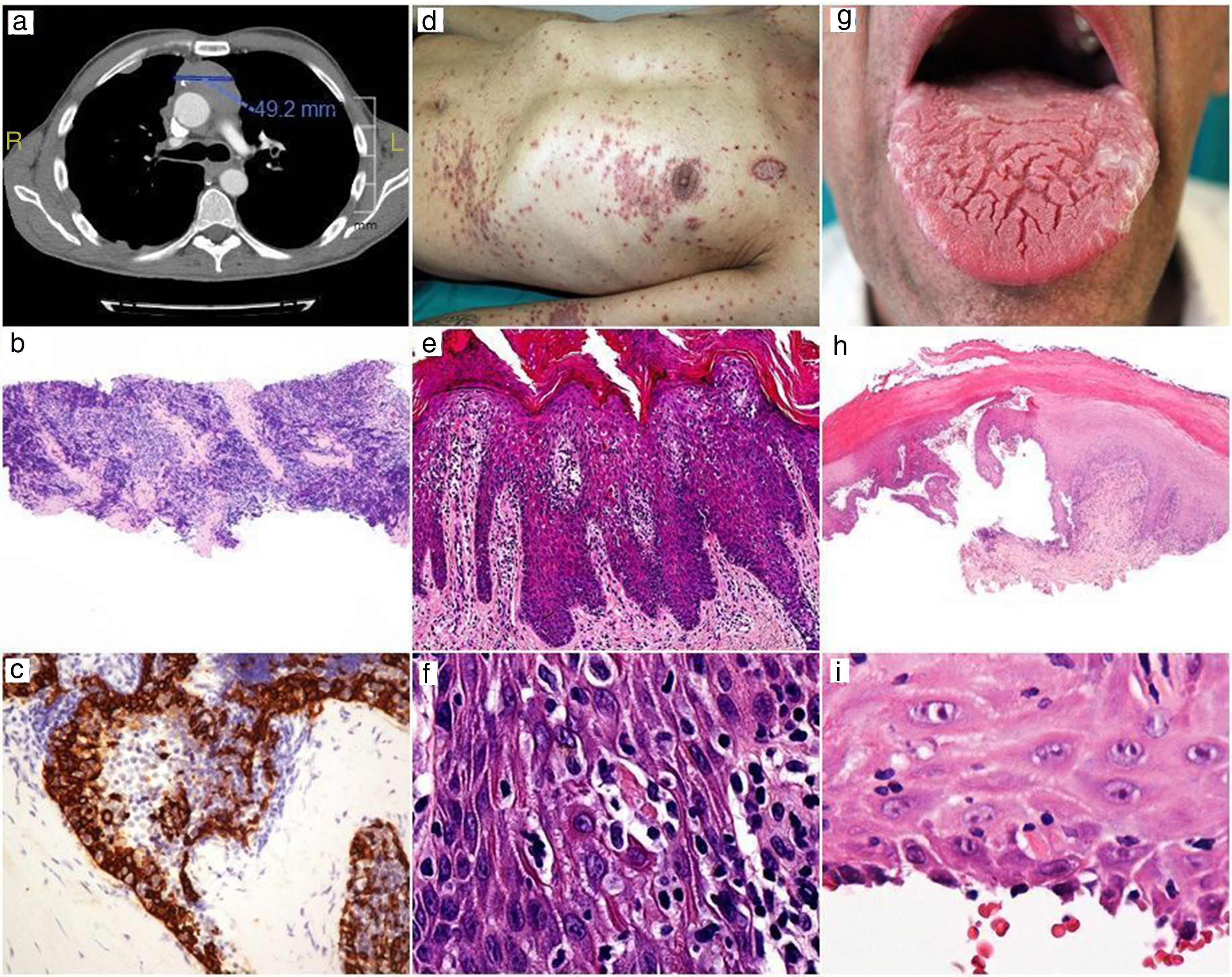
CT showing thymoma tumor mass and lung metástasis (a). Low-power view of thymoma needle biopsy with a predominant small cell population, H-E x20 (b). Higher magnification showing cytokeratin-positive larger epithelial tumor cells and numerous lymphocytes, AE1-AE3, x400 (c).
Skin rash (d). Epidermal hyperplasia with numerous apoptotic keratinocytes, H-E x200 (e). High power showing apoptotic keratinocytes with satellite lymphocytes, H-E x400 (f).
Oral lesions (g). Scanning magnification showing bullous lesion with extensive basal cell damage, H-E x20 (h). The roof shows apoptotic keratinocytes with satellite lymphocytes, H-E x400 (i).
Laboratory tests showed no liver or renal anomalies, no hypogammaglobulinemia, but mild positive anti-nuclear and double strand-DNA autoantibodies. Endoscopic findings were submucosal hemorrhages. Stool exams were negative.
Cutaneous and mucosal biopsies were performed. Histopathology of the skin lesion revealed moderate epidermal hyperplasia with parakeratosis and an interface dermatitis with numerous apoptotic epidermal keratinocytes, some of them with satellite lymphocytes. The underlying dermis showed mild lymphocytic perivascular infiltrate. Intraepidermal lymphocytes were predominantly CD4-positive, with a minor component of CD8-positive cells. These findings were consistent with TAMA. Mucosal biopsy was consistent with erosive oral lichen planus. (Fig. 2 b, c, e, f, h, i).
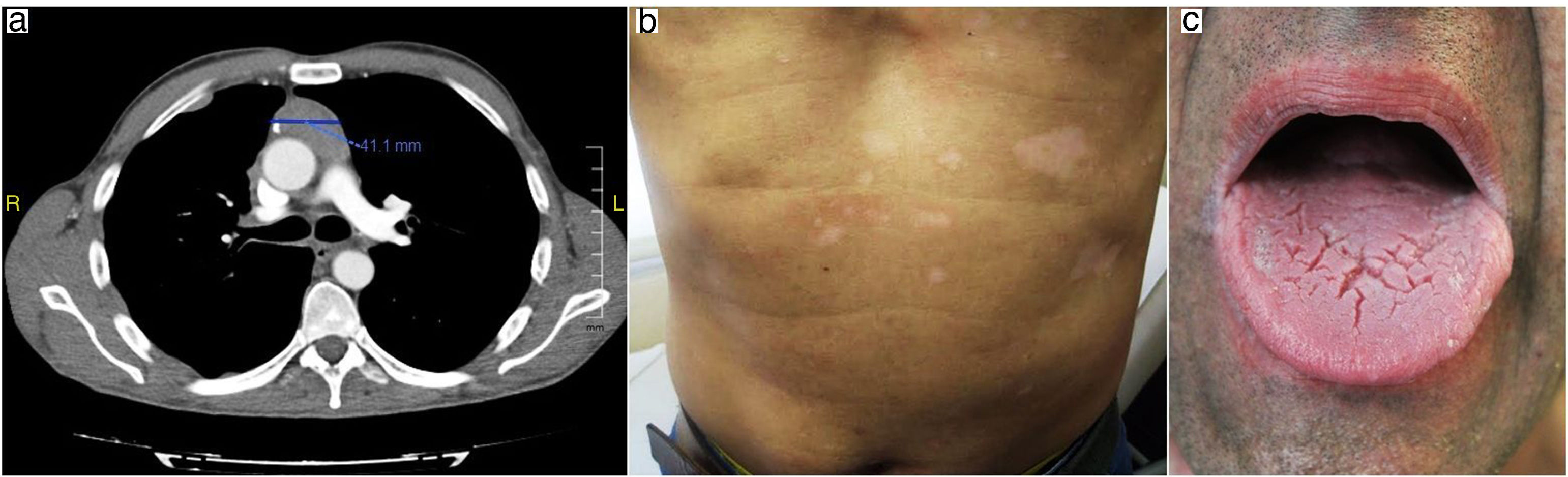
Oral prednisone (30mg/day) was initiated with slow improvement of the lesions and relapse after dose reducing. Mucosal lesions quickly improved after two months with topical 0.0001% tacrolimus solution two times a day. Cyclosporine was removed and computerized tomography showed decrease in thymoma tumoral mass despite treatment with corticosteroids as monotherapy for a year. (Fig. 3 a, b, c).
Thymoma-associated multi-organ auotimmunity (TAMA) is a term fisrt proposed by Wadhera et al in 2007 which describes a multi-organ disease (mainly affecting the liver, intestine and skin) in the setting of a malignant thymoma with clinical and histological findings overlapping those of the graft-vs-host disease but in the absence of hematopoietic transplantation.5 To our best knowledge there are only abut 30 cases of TAMA published in the current literature and it mainly affects middle-aged patients of both sexes with slight female predominance.6 The involved pathogenesis in TAMA is similar to that present in GVHD, so defective T cells originated in a damaged thymus would be unable to differentiate self from foreign antigens, leading to a loss of self-tolerance. Cutaneous lesions in TAMA include an extensive erythematous maculopapular or psoriasiform rash with more or less hyperkeratosis, which can involve the whole body. Differential diagnosis may include true graft-vs-host disease, viral infections, toxicodermia and post-transfusion reactions. TAMA has often a bad outcome, mainly due to opportunistic infections.5–7 Moreover, autoinmune disease other than myasthenia gravis are frequently associated with an advanced Masaoka thymoma stage.1
Lichen planus has been reported previously as an infrequent finding in patients with thymoma, often found in the context of TAMA and not as an insolated disease. Most cases show an aggresive oral erosive lichen planus which tends to be refractory to treatment and thymectomy does not seem to be effective. Our patient showed an early response to low concentration tacrolimus solution.1,2,8
Multiple systemic treatments (corticosteroids, retinoids, cyclosporine, phototherapy…) have been used in the management of skin lesions associated to thymoma with differents results, however immunosupressive treatments have been related with an increased risk of infections and death. Some authors propose that the treatment must be focused, if possible, on the therapy for thymoma, trying complete resection of the tumour; while others suggest that thymectomy can worsen the course of the autoimmune disease.1,2,9,10
In summary, we report a new case of an advanced-stage malignant thymoma associating multiple paraneoplastic syndromes such as TAMA (with skin rash and diarrhoea), erosive oral lichen planus treated with topical tacrolimus, bone narrow aplasia, and positive ANA and ds-DNA autoantibodies. Additionally, despite of unresectable thymoma, the patient is showing an improvement of both tumour and cutaneous rash with the use of prednisone as monotherapy.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sánchez-Pérez S, Monteagudo-Castro C, Martín-Hernández JM, Ramón-Quiles MD. Timoma de estadio avanzado asociado a síndromes paraneoplásicos con buena respuesta a los corticosteroides orales y tacrolimus tópico. Actas Dermosifiliogr. 2019;110:60–62.