Nivolumab is a Programmed Death-1 (PD1) receptor (PD-1) inhibitor, first approved as cancer immune-checkpoint inhibitor therapy for stage IV malignant melanoma, with recent indications in different tumors such as non-small cell lung cancer, prostate cancer, renal-cell cancer or ovarian cancer. A wide variety of skin reactions have been described associated to cancer immunotherapy.1–7 We present a case of morphea relapsing during Nivolumab treatment.
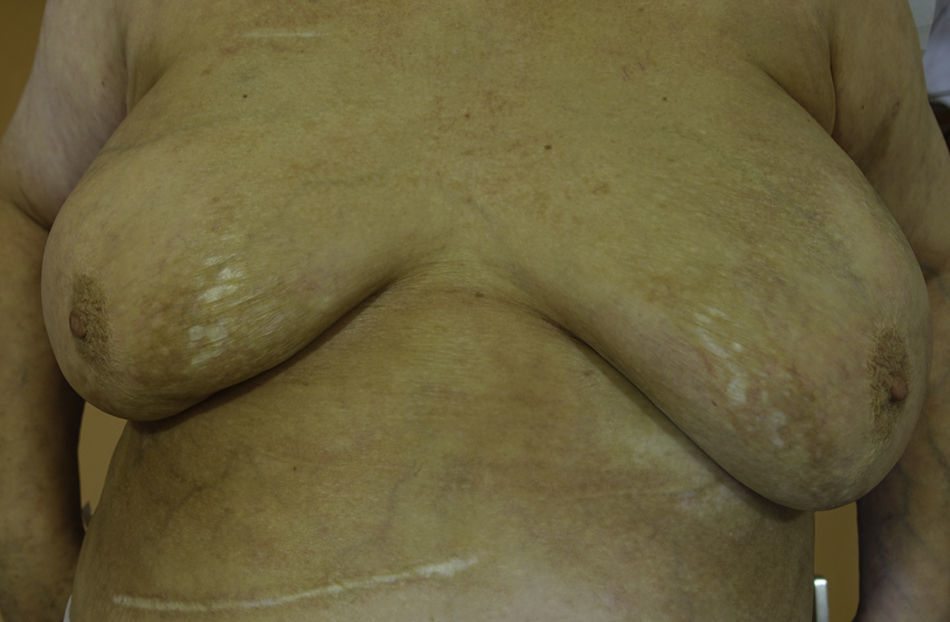
A 65 year-old woman came to our clinic referred by the Oncology department of our hospital. She was being followed due to a stage IV lung adenocarcinoma for which she had received different therapies. Just 2 months after initiating treatment with Nivolumab (3mg/kg every 2 weeks), she developed cutaneous lesions on the trunk. On the physical examination she presented three patches: one on each breast, and one on the left inframammary fold (Fig. 1). These lesions had a shiny white color with a discrete lilaceous ring. To the touch, they presented as clearly atrophic. She reported occasional itching but no other symptoms. The patient had a past medical history of morphea 8 years before (histologically diagnosed), having been treated with topical corticosteroids and oral methotrexate, with remission of the disease for more than 6 years. Postinflammatory hyperpigmentation was not detected.
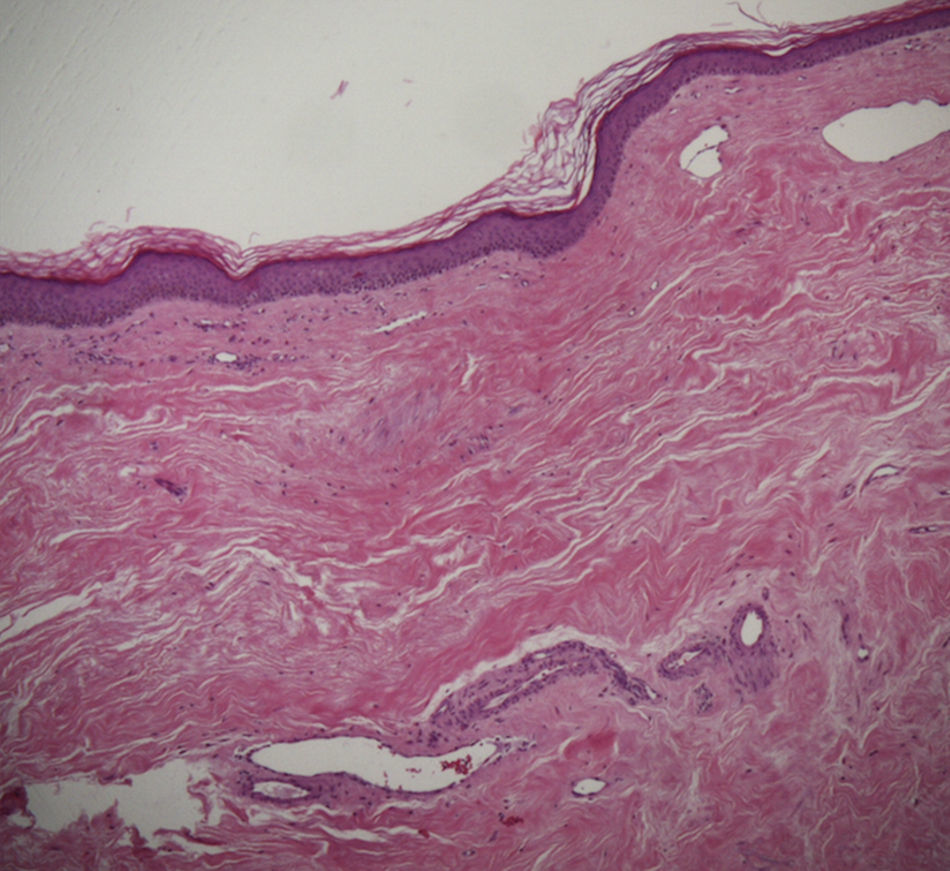
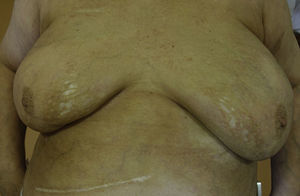
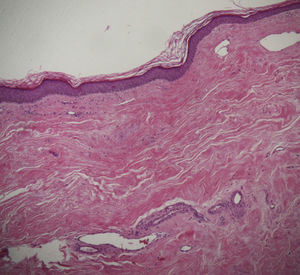
A skin biopsy of one of the patches was performed. Histological examination demonstrated findings of lichen sclerosus with underlying morphea (Fig. 2). Nivolumab was stopped after 6 months of treatment because of the lack of efficacy in oncological terms. In subsequent visits to our clinics, the patient showed improvement of her cutaneous lesions, without any topical or systemic treatment.
Relapse of morphea has been described in 50% of patients in the first 2 years after diagnosis.8 In the presented case, more than 6 years had passed since the last exacerbation of morphea, and this episode of relapsing happened only 2 weeks after initiating Nivolumab. Although natural evolution of the disease may have played a role, we considered Nivolumab as a possible trigger.
Many other medications have been associated with the development of morphea. The formation of autoantibodies and subsequent microvasculature injury has been proposed in the pathogenesis of these cases of drug-induced morphea.9
In different clinical trials, anti-PD1 therapies such as Pembrolizumab or Nivolumab showed a relatively safe profile, with a low incidence of major adverse effects (drug-related grade 3 or 4 toxic effects only in 14% of the patients), being those related with pneumonitis the most severe of them.10 These adverse reactions related with immune-checkpoints inhibitors have been named as immune-related adverse events (irAEs). When focusing on the skin irAEs, non-specficic macular papular rash and pruritus have been described as the most common ones. Interestingly, vitiligo has also been reported, but only in patients with melanoma. Urticaria, alopecia and mucosal involvement are other frequently described skin irAEs.1–4
The exacerbation of preexisting autoimune disorders related to immunotherapies has also been reported. These disorders include psoriasis, sarcoidosis, bullous pemphigoid and subacute lupus erythematosus.5–7 To the best of our knowledge, this would be the first case of morphea relapsing due to any cancer immunotherapy treatment.
In conclusion, attending to the nature of this kind of immunotherapies, totally different from traditional chemotherapy, dermatologists should be aware not only of their typical irAEs, but also of the possibility of exacerbation or relapse of previously controlled skin diseases, especially immune-related ones. This will probably represent a new challenge for dermatologists in the future, as these treatments are being used more and more often. More experience is needed in order to conclude the exact relation of cancer immunotherapy and the relapse of these diseases.
Conflict of interestsThe authors declare no conflict of interest.
A C. M. García del Real for his collaboration.