Over the past few years, venereal or sexually transmitted infections (STIs) have been on the rise worldwide requiring additional specialized monographic consultations to specifically treat STIs. Therefore, the Spanish Academy of Dermatology and Venereology (AEDV) Research Working Group on STIs and HIV has drafted this document with the necessary requirements in terms of infrastructure, personnel, technology, specific materials for sample collection, and needs for current therapeutic options. Strict emphasis is placed on the protection of patient privacy. A health care circuit model is outlined too. Additionally, a section has been included on contact tracking and reporting, key elements for the effective prevention and control of STIs. These clinical practice guidelines seek to establish a clinical action framework adapted to the current challenges posed by STIs and HIV in the dermatology, venereology, and multidisciplinary settings.
El incremento de infecciones venéreas o de transmisión sexual (ITS) es un fenómeno global que requiere especial atención. Ante esta situación, y al reconocer la necesidad de establecer consultas monográficas, desde el Grupo Español de Investigación en ITS y en virus de la inmunodeficiencia humana (VIH) de la Academia Española de Dermatología y Venereología (AEDV) se desarrollan en este documento los requisitos necesarios en términos de infraestructura, personal, tecnología, materiales específicos para la recolección de muestras y las necesidades terapéuticas actuales. Se destaca la importancia de la protección de la privacidad de los pacientes, y se delinea el circuito asistencial recomendado. Además, se incluye una sección dedicada a la trazabilidad y notificación de contactos, elementos clave para la prevención y control efectivos de estas infecciones. Estas directrices buscan establecer un marco de actuación clínico, ajustado a los desafíos actuales que presentan las ITS y el VIH en el ámbito dermatovenereológico y multidisciplinar.
Venereal or sexually transmitted infections (STIs) have experienced a tremendous increase in the past decade, making it more necessary than ever to reorganize care pathways in response to the existing high demand.1 For this purpose, we consider the formation of multidisciplinary groups of paramount importance, which should ideally be arranged into units, sections, or monographic centers, to provide better care for patients. The benefits of integrating different specialties contribute to the health care center where the activity is conducted, the health care workers, and the patients alike. Firstly, for the health care center involved, the addition of different specialties leads to a more comprehensive and coordinated care (with fewer follow-up visits), and not so many unnecessary additional tests. Secondly, for the health care workers involved, it means being able to acquire better knowledge on a continuous learning basis, with a greater sense of belonging, and the possibility of developing their own research lines. Thirdly, it is safer for both the patients and the health care workers, as all decisions are agreed upon, especially in complex situations. Lastly, for the patients, it is a comprehensive, holistic, and effective care tailored to their actual health needs, with the possibility of being referred to the appropriate specialist.
Thanks to the involvement of different professionals, medical education, prevention, diagnosis, both the treatment, and management of complications arising from the existence or risk of venereal infections can be addressed.
Legal framework and general ethical considerationsThe corresponding guide addresses both the ethical considerations and legal framework. This article sheds some light on the fact that the organization of the monographic consultation room must be focused on providing excellent care in line with the legal and ethical standards of the patients’ backgrounds.
Minimum human resourcesThe following resources are required for the basic management of patients with or at risk of STIs:
- -
Administrative staff: aware of the privacy required in the management of this group of diseases.
- -
Trained nurses: they play a key role in the screening of patients, medical education on STI prevention, as well as on the administration of therapies and sample collection.
- -
Laboratory technicians: woking at the clinical analysis/microbiology unit.
- -
Microbiologists: who should be coordinated for issuing results and discussing inconclusive results with the clinician, etc.
- -
Medical specialists in dermatovenereology, gynecology, urology, family and community medicine, internal medicine, etc.
When initiating activity in a health care center where STIs will be addressed, the special need for the patients’ privacy and confidentiality must be considered. An exclusive or special waiting room is not deemed necessary. However, special consideration must be paid to some particularities such as avoiding to explicitly announce the name of the disease at stake (as in the case of venereal infections) when exiting the office. Ideally, in full compliance with privacy recommendations, a call system to the consultation room with an anonymous code that without any patient identification data should be available, rather than a voice call by first and last name. Beyond this circuit aspect, the consultation or visit room per se should include the following features:
- -
Door with a system to minimize all possible interruptions during the clinical visit from the outside.
- -
Possibility of direct or close access to a toilet for self-sampling, if necessary.
- -
Furniture:
- ∘
Support desk for computer tools.
- ∘
Chairs and stools for the health care workers.
- ∘
Seats with room for a companion.
- ∘
Reclining examination bed.
- ∘
Floor or ceiling lamp for examination.
- ∘
Cart or cabinets with the necessary laboratory material for sample collection, and the different treatments available. The examination bed needs to be in a separate space, or isolated from the the rest of the room with a curtain or screen in such a way that the physical examination can take place in an atmosphere of privacy and trust in the health care workers. The entrance door to the office should not be directly visible from the examination room. In other words, visual and acoustic privacy of the office should be observed at all times.
- ∘
Coat rack or other furniture to place the patient's clothes when necessary.
- ∘
Storage room for material for physical examination: gloves, mask, if necessary, dermatoscope, etc.
- ∘
Optional material: microscope in the same office, techniques to perform point-of-care tests (those which are performed in the same patient care location and allow results in a short time such as minutes, or just a few hours).
- ∘
Basic needs include:
- -
A laptop or desktop computer to register patients, fill out the patients’ health records, prescribe treatments to be administered at the center, sign prescriptions, etc. This equipment should also allow anonymous calls to be made through systems such as the code alert on the screen indicating where the patient should go (after registering and receiving the corresponding code).
- -
A phone or similar resource to conduct non-face-to-face visits and follow-ups and be able to call other professionals from the center.
- -
Under ideal conditions, handheld computers (tablets) and smartphones (with exclusive office lines) should be available to:
- ∘
Speed up result notification to patients.
- ∘
Aid in tracing systems and contact notification.
- ∘
Facilitate the collection of sociodemographic data, sexual practices, etc.
- ∘
For sample collection and treatment administration in the consultation, the following should be made available (table 1).
Necessary material for sample collection and treatment administration in the clinic.
| A) Personal protective equipment |
| - Gloves (preferably nitrile) |
| - Disposable splash-proof gown (waterproof) |
| - Masks (at least, surgical) |
| - Eye protection (if feasible) |
| B) Sample collection material |
| - Extraction needles with butterfly, syringe, and tube |
| - Safety lancet |
| - Compressor |
| - Aqueous 2% chlorhexidine |
| - Cellulose dressings |
| - Tubes for blood collection/extraction with different additives: |
| * With gel or no additive for parameters measured in serum (serology, biochemistry, hormones, etc.) |
| * With lithium or sodium heparin (for certain biochemistry and serology tests) |
| * With ethylenediaminetetraacetic acid (EDTA) anticoagulant (for blood and plasma tests) |
| * With sodium citrate (potentially for coagulation tests) |
| - Tubes for swabs and samples for polymerase chain reaction (PCR): |
| * With a culture medium: |
| * For aerobic germs: tube with a cap equipped with swab rod, with semisolid transport medium |
| * For viruses, Chlamydia, and Mycoplasma/Ureaplasma: tubes with media such as the Universal Transport Medium (UTM® [Becton Dickinson, Inc., United States, but also available from other manufacturers]), which allow maintaining virus viability for more than 72 hours |
| * Without a culture medium: often tubes with a cap equipped with a swab rod |
| - Petri dishes with the following culture media for the growth and development of different microorganisms: |
| * Modified Thayer-Martin (equivalent to vancomycin, colistin, amphotericin, and trimethoprim [VCAT]): selective medium for isolation and growth of Neisseria bacteria |
| * Agar-chocolate PolyViteX™ (Biomérieux, Inc., France; or an alterlative of similar characteristics): for microorganisms with special requirements, suitable for growth of Neisseria and Haemophilus bacteria |
| * Blood agar: conventional growth medium for different microorganisms |
| * Candida agar: selective growth of Candida yeast |
| * Gardnerella agar: selective isolation of Gardnerella vaginalis |
| * Roiron: For selective growth of pathogenic species of Trichomonas and Candida2 |
| - Microscope slides and coverslips for fresh sample microscopic examination |
| - Methylene blue for direct examination of urethral samples |
| - Serum and potassium hydroxide for direct examination of vaginal samples |
| C) Material for treatment administration |
| - 5 mL and 10 mL syringes |
| - Needles for loading medication (21 G gauge) |
| - Intramuscular needles (21 G x 40 mm) |
| - Ampoules of physiological saline solution |
| - Ampoules of 1% lidocaine |
| - Medication ampoules: ceftriaxone 500 mg/1000 mg with its solvent, penicillin G-benzathine of 2.4 million IU, etc. |
| D) Material for rapid HIV testing |
| - Rapid HIV tests on capillary blood |
| - Capillaries with EDTA |
| - Disposable sterile 23 G safety lancets |
| Chase buffer (buffer solution) |
| E) Material for biopsies |
| - Ampoules of 1% lidocaine or 2% mepivacaine |
| - Scalpels with no. 15 blade/punches (circular scalpels) for biopsy sampling |
| - Surgical material for biopsy suturing |
| - Microscope slides and coverslips for Tzanck test or similar technique examination |
| - Bottles with formalin for sample transportation and processing in pathology |
EDTA, ethylenediaminetetraacetic acid; HIV, human immunodeficiency virus; PCR, polymerase chain reaction; VCAT, vancomycin, colistin, amphotericin, and trimethoprim.
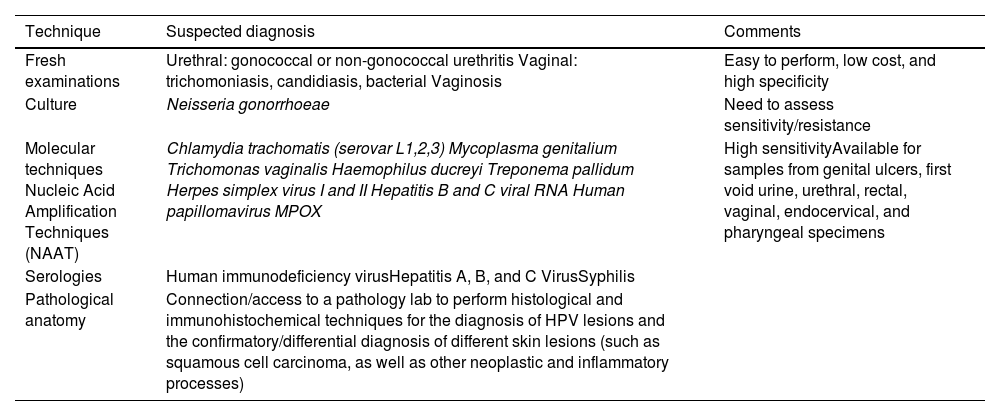
For the diagnosis of STIs in a partner lab associated with the monographic consultation, various diagnostic methods must be available, which we discuss below and summarize in table 2.
Summary of the techniques that a partner lab associated with the sexually transmitted infections clinic should have.
| Technique | Suspected diagnosis | Comments |
|---|---|---|
| Fresh examinations | Urethral: gonococcal or non-gonococcal urethritis Vaginal: trichomoniasis, candidiasis, bacterial Vaginosis | Easy to perform, low cost, and high specificity |
| Culture | Neisseria gonorrhoeae | Need to assess sensitivity/resistance |
| Molecular techniques Nucleic Acid Amplification Techniques (NAAT) | Chlamydia trachomatis (serovar L1,2,3) Mycoplasma genitalium Trichomonas vaginalis Haemophilus ducreyi Treponema pallidum Herpes simplex virus I and II Hepatitis B and C viral RNA Human papillomavirus MPOX | High sensitivityAvailable for samples from genital ulcers, first void urine, urethral, rectal, vaginal, endocervical, and pharyngeal specimens |
| Serologies | Human immunodeficiency virusHepatitis A, B, and C VirusSyphilis | |
| Pathological anatomy | Connection/access to a pathology lab to perform histological and immunohistochemical techniques for the diagnosis of HPV lesions and the confirmatory/differential diagnosis of different skin lesions (such as squamous cell carcinoma, as well as other neoplastic and inflammatory processes) |
HPV, human papillomavirus; NAAT, nucleic acid amplification techniques.
Microscopyandcultures are routine determinations in symptomatic patients. Direct examinations for the visualization and diagnosis of Trichomonas vaginalis are easy to perform, inexpensive, and highly specific, although low sensitivity rates have been reported. A rapid and sensitive technique that also requires the use of a microscope and has been indicated in cases of symptomatic urethritis is Gram staining or toluidine blue. It is not recommended in rectal or pharyngeal samples. In urethral samples of symptomatic male patients this technique is associated with high sensitivity rates (from 90% up to 95%), which drop in asymptomatic men though (from 50% up to 75%). Regarding chlamydia infections, specificity rates are low. Culturing exudates, prior to treatment, is important in the case of gonococcus for conducting antibiotic sensitivity testing and studying antibiotic sensitivity to learn about resistances and the appropriate treatment.3
Methods based on nucleic acid amplification techniques (NAATs) are more sensitive for the diagnosis of Chlamydia trachomatis, Mycoplasma genitalium, and T. vaginalis, and have demonstrated a greater sensitivity than culture in the diagnosis of Neisseriagonorrhoeae. These microorganisms can be diagnosed in samples from vaginal, endocervical, urethral, rectal, and pharyngeal swabs and first morning urine. NAATs exist in real-time multiplex polymerase chain reaction (PCR) platforms, both in point-of-care and automated systems for multiple samples.4,5 This same methodology, NAAT, is used to detect etiological agents causing genital ulcers due to the low sensitivity of microscopy and the longer time required for diagnosis with cell cultures. Various systems with multiple formats are currently available for the identification of different microorganisms in a sample such as Haemophilus ducreyi, Treponema pallidum, C. trachomatis of lymphogranuloma venereum (LGV) serotypes, or herpes simplex virus types 1 and 2. Lesions with suspected human papillomavirus (HPV) etiology do not always require microbiological study. However, in lesions of uncertain significance in cytology or in the clinical course, a PCR for HPV diagnosis and its genotype is advised.6–9 In the case of syphilis diagnosis, PCR is a useful technique in patients with suspicious but serologically non-reactive lesions (primary syphilis) and oral, rectal, and exudative lesions. However, indirect serological methods based on antibody detection have shown excellent results for syphilis diagnosis. These include nontreponemal tests such as rapid plasma reagin (RPR) and venereal disease research laboratory (VDRL) and treponemal tests such as enzyme immunoassay (EIA), Treponema Pallidum hemagglutination assay (TPHA), Treponema Pallidum particle agglutination assay (TPPA) or chemiluminescent immunoassay (CLIA). The latter are automated and used at the beginning of the syphilis diagnostic algorithm as they are more specific than nontreponemal tests.10
Serological methods are used for the diagnosis of human immunodeficiency virus (HIV) and hepatitis A (HAV), B (HBV), and C (HCV) viruses. HIV determination is based on automated immunoassay techniques (enzyme-linked immunosorbent assay [ELISA], EIA, chemiluminescent immunoassay [CLIA]), which are cost-effective in high-volume laboratories. The most recent techniques (4th generation) simultaneously detect antibodies and protein 24 (p24) antigens, which increases their sensitivity while reducing the possibility of false negatives. There are rapid techniques (< 30 minutes) that do not require any immunochromatography-based devices for a preliminary diagnosis of HIV. These tests are not as sensitive as 4th generation immunoassays for the diagnosis of acute infection. Immunoassay-based confirmatory diagnostic tests such as western blot (WB) or immunochromatography containing specific recombinant or synthetic peptides for HIV-1 (glycoprotein 160 [gp160], 41 [gp41], protein 31 [p31], p24) and HIV-2 (glycoprotein 140 [gp140], 36 [gp36]); their reading and interpretation are automated. Another technique included in this diagnostic algorithm involves the detection of viral RNA by NAAT in cases of indeterminate or discrepant serology.11 The microbiological diagnosis of HCV is based on the demonstration of anti-HCV antibodies or antigens by enzyme immunoassay and detection of viral RNA by NAAT that allow the differentiation of disease stages. For HBV detection, analyzes are based on serological tests aimed at detecting classic serological markers such as hepatitis B surface antigen (HBsAg) and specific antibodies, as well as NAAT methods for the detection and quantification of viral DNA. Immunoassays are also used for the diagnosis of immunoglobulin M (IgM)-HAV antibodies as a serological marker of acute infection.5,12
Organization and patient flowIdeally, patients should have the possibility of scheduling directly in STI consultation rooms. Although this is an easier thing to do in monographic STI units or centers, it may also be reasonable for patients to be referred from other departments or services (e.g., in a hospital, from emergency services, primary care, gynecology, internal medicine, etc.). If these consultations are not integrated into the same center, service, section, or unit, the dermatovenereologist must be able to refer the patient to other specialties in cases that need it (e.g., a patient with a recent HIV diagnosis who requires initiation of antiretroviral therapy).
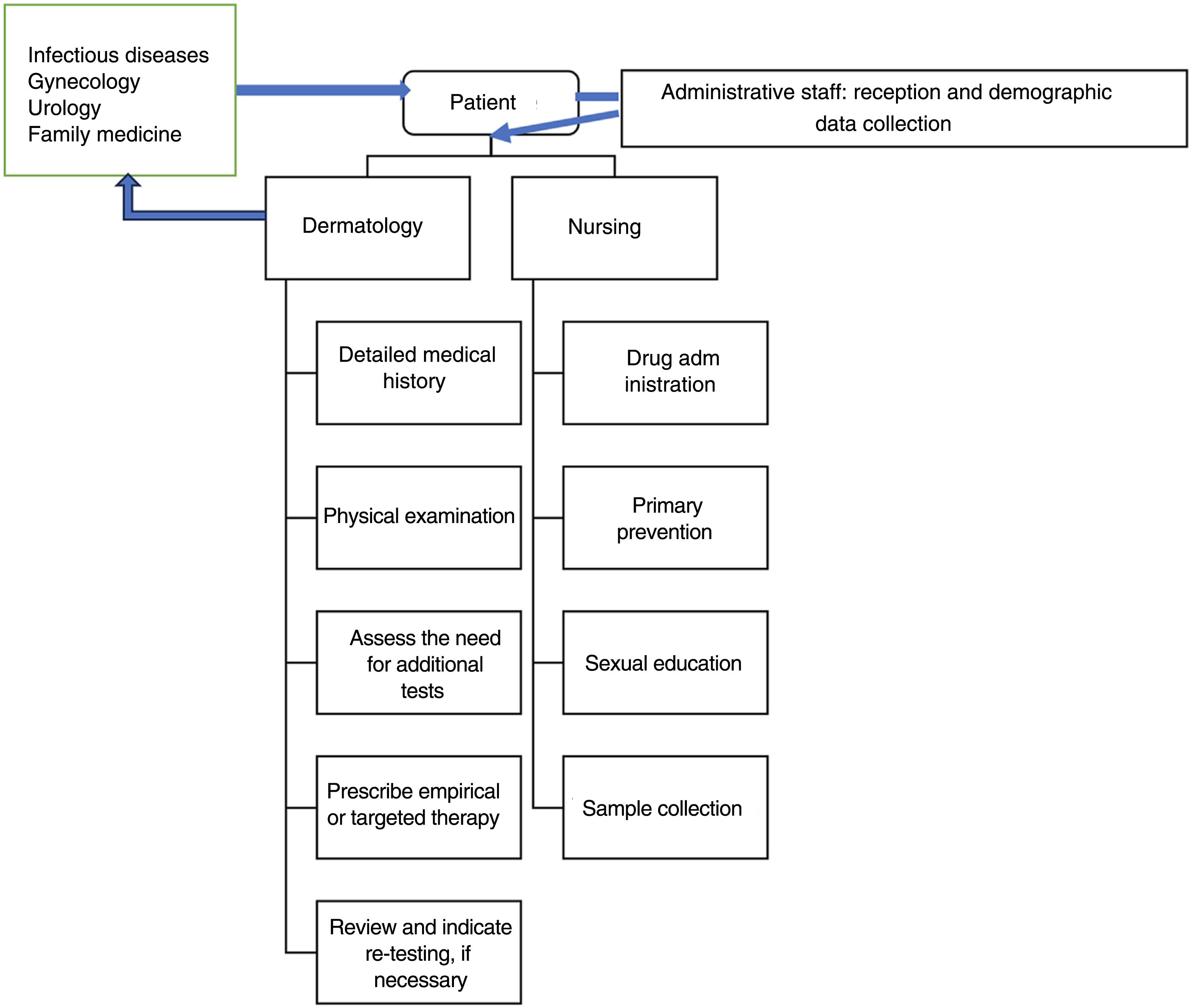
These consultation rooms be part of a multidisciplinary center with medical specialties other than medical-surgical dermatology and venereology (such as gynecology and obstetrics, urology, internal medicine, family and community medicine), as well as other health care workers (such as psychologists, social workers, social educators, etc.).13 However, in the model presented here, the dermatovenereologist is at the center of the organization, because in Spain his is the only specialty that includes venereology in its name, and the only with ad hoc training in this field.
Nurses deserve special consideration. In addition to contributing to sample collection, they also contribute to areas such as continuous medical education. Additionally, in some centers, the management and request of tests for asymptomatic users who come for screening are conducted by advanced practice nursing.
Data protection and confidentiality13 are of paramount importance in an STI consultation room and must be observed from the first contact with the patient. The initial reception (figure 1) will be sole responsibility of the administrative staff while screening, in some cases, will be performed by the nurses. The use of anonymized codes to register patients helps manage health records and preserve confidentiality. The patient's privacy must be respected at all times while providing the patient with a separate place to dress and undress, and the possibility to be accompanied by a relative or friend. The goal is to create a favorable environment that facilitates obtaining a comprehensive and detailed medical history and explaining the justification of the questions asked naturally to better assess the patients’ risk and direct tests appropriately.10
In centers with health care workers in training, it is advisable to inform the patient about their presence and obtain their verbal consent prior to their examination, so that they do not feel uncomfortable.
There are different systems for notifying results and follow-up steps after the initial visit, depending on the capabilities of each center:
- -
In-person visits.
- -
Phone calls.
- -
Message sending to mobile phones (with prior authorization) with limited but useful content, without confidential data.
- -
Notifications through apps requiring a username and password (already implemented by some centers or autonomous communities for result consultation).
Since single-dose drugs are already available for the management of many STIs, and treatment is preferably administered in the clinic to promote compliance to it and healing, the following drugs are recommended:4,14
- -
Benzathine penicillin G 2,400,000 IU intramuscularly (IM), as the first-line therapy for syphilis (primary, secondary, and early latent with 1 dose; late or indeterminate latent with 3 doses 1 week apart to reach 7.2 million IU).14,15
- -
Azithromycin 1 g orally (PO), as the first-line therapy to treat chancroid and donovanosis, and cases of chlamydia where doxycycline is ill-advised.
- -
Ceftriaxone 500/1000 mg ampoules for IM application to treat gonococcal infections (1 g in patients > 150 kg in weight) and chancroid.
- -
Gentamicin 240 mg IM, associated with azithromycin 2 g PO to treat gonorrhea in patients allergic to cephalosporins.
- -
In indicated cases where post-exposure prophylaxis (PEP) for HIV14 is required (e.g., tenofovir disoproxil fumarate, emtricitabine plus raltegravir or dolutegravir). Overall, the recommended regimen is 28 days and it should be initiated within 72 hours of exposure.16
- -
Pre-exposure prophylaxis (PrEP) for HIV (mainly done with emtricitabine/tenofovir) will be administered and can be initiated after the consultation, in case of new diagnoses of syphilis, with negative HIV serology, and in individuals at risk of acquiring HIV.17
- -
Additionally, ablative techniques for treatment in the clinic should be available in case of genital warts or contagious mollusks, such as cryotherapy, 80% to 90% trichloroacetic or dichloroacetic acid, or an electrocoagulator.
We briefly summarize below the steps that should be taken for the contact tracing of a person diagnosed with a venereal infection:18
- 1)
Explain the reasons for seeking contacts: Patients should be informed that:
- -
Sexual contacts may be asymptomatic and unaware of their infection.
- -
If sexual partners are not informed, examined, or treated, serious complications can occur.
- -
If the sexual partner does not receive proper treatment, there may be a risk of reinfection upon contact again.
- -
- 2)
Help identify who needs to be notified:
- -
Addressing the mode of transmission and the likely duration of the infection.
- -
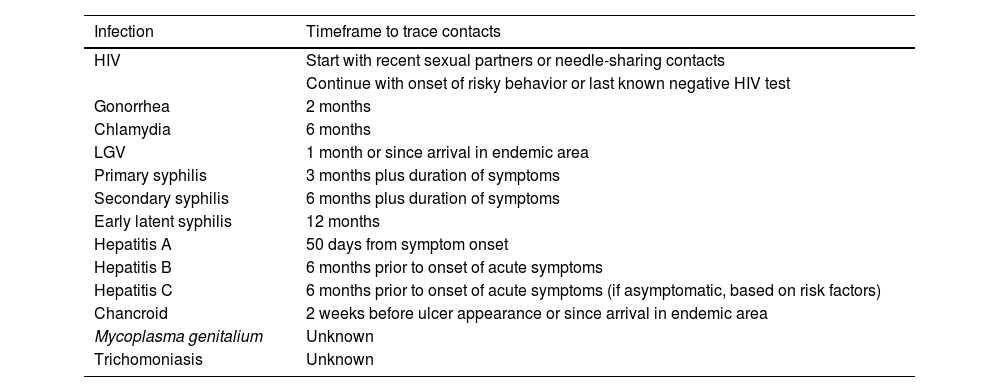
Contact tracing from relevant time periods in each specific case (table 3).
Table 3.How far back should contact tracing go in the diagnosis of venereal infections?20
Infection Timeframe to trace contacts HIV Start with recent sexual partners or needle-sharing contacts Continue with onset of risky behavior or last known negative HIV test Gonorrhea 2 months Chlamydia 6 months LGV 1 month or since arrival in endemic area Primary syphilis 3 months plus duration of symptoms Secondary syphilis 6 months plus duration of symptoms Early latent syphilis 12 months Hepatitis A 50 days from symptom onset Hepatitis B 6 months prior to onset of acute symptoms Hepatitis C 6 months prior to onset of acute symptoms (if asymptomatic, based on risk factors) Chancroid 2 weeks before ulcer appearance or since arrival in endemic area Mycoplasma genitalium Unknown Trichomoniasis Unknown HIV, human immunodeficiency virus; LGV, lymphogranuloma venereum.
- -
Conducting a thorough sexual history:
- ∘
It should be investigated whether the contacts can be located and how the patient feels about contacting them personally.
- ∘
Resources for contact tracing should be offered.
- ∘
Some additional considerations need to be made:
- ∘
The index patient may have had casual or anonymous partners and may not know their identities or contact information.
- ∘
It may not be necessary to exhaustively ask for all details of each partner; however, in the case of more serious infections, such as HIV, a more detailed partner history is justified.
- ∘
A more detailed inquiry could include explicit information on the relationship with the contacts, specific sexual practices, condom use, physical location, transactional sex, etc.
- ∘
For bloodborne infections, inquiries should be made about blood donation, receipt of blood products, and exposure to sharps.
- ∘
- -
- 3)
Explain the available methods and proceed with contact tracing:
Contacts can be notified through patient- or provider-initiated contact tracing. It is essential to work together with the patient in full compliance with the current legislation (specifically, the Organic Law No. 3/2018, of 5 December 2018, on the Protection of Personal Data and the Guarantee of Digital Rights)19 and the characteristics of our environment.
Patient-initiated contact tracing involves the index patient notifying their contacts of the diagnosis. The health care worker provides the information the index patient needs to transmit to his/her partners. It has 2 advantages: (1) it is almost always more acceptable than professional-initiated tracing, and (2) it is faster and easier to reach established anonymous contacts through social media or dating apps. It has some disadvantages, such as
- (1)
the potential loss of patient confidentiality,
- (2)
depends on the patient's intentions, and
- (3)
the possibility of incomplete or incorrect communication of information.
Provider-initiated contact tracing involves the health care professional after receiving authorization from the patient and maintaining strict confidentiality, directly notifying contacts, or resorting to another organization to make sure they are notified. In some cases (such as diagnoses of primary HIV infection), follow-up with the index patient may be necessary to assess how contact tracing has progressed, also counting on their help and support if needed. Pros: it usually maintains the confidentiality of the index case and provides protection against violent or stigmatizing reactions due to anonymity. Cons: it takes more time and resources, and there is a possibility that the index patient will not be disclosing all their contacts to the health care worker.
- (1)
- 4)
Inform the contacts:
Through various channels: in person, via phone call/text message, through social media, with web tools or notification apps (in our country, there have been several attempts, being the most notable example (though not used for venereal infections) the Radar COVID application during the SARS-CoV-2 pandemic, without achieving effective tools that can be used), via e-mail, letter, or through referral to a center or specialist.
Overall, contact tracing in Spain is an aspect that needs improvement in our activity, and the Spanish Academy of Dermatology and Venereology (AEDV) has been making efforts to find additional solutions beyond the voluntarism of the patients who consult us (which is currently where most of the contact tracing falls).
This section will depend on the organizational characteristics of each Service and Center. We believe that, under ideal conditions, there should be fluid communication and links with:
- -
Primary care physicians and midwives: for receiving patients through a quick referral circuit.
- -
Other medical specialists: microbiologists, clinical analysts, internists, etc., for all situations where their participation or assistance may be necessary.
- -
Social workers: to assist with social/socio-health aspects.
- -
Various socio-health resources (NGOs and their checkpoints, centers for drug addiction help, etc.), which will allow for a complementary approach to the needs of our patients.
Currently, venereological care in Spain is predominantly provided outside conventional dermatology services.21 This, together with a clear increase in STI cases in recent years,22 makes it imperative to rethink the organization of the health care provided to patients with or at risk of venereal infections. In the preceding pages, we tried to systematize the recommended characteristics of monographic units or centers. We did so convinced that adequate organization and planning of services (ideally monographic, led by specialists in venereology and multidisciplinary) allow for maximizing the reach, effectiveness, and efficacy of health care and preventive resources, ultimately contributing to controlling this concerning current epidemiological trend.
FundingNone declared.
Conflicts of interestNone declared.