Intravenous immunoglobulin (IVIG) replacement therapy has been used in immune deficiency diseases for more than 50 years. The indications for this treatment have evolved, however, and IVIG therapy is now used in various diseases in which the immune system plays a prominent role. IVIG therapy has carved out a niche in dermatology for the treatment of such conditions as dermatomyositis, autoimmune bullous diseases, and toxic epidermal necrolysis. Special attention has been paid to this therapy in recent years. New guidelines have been published and should be taken into consideration in dermatology. This review provides a practical guide to IVIG use in our specialty.
El uso de las inmunoglobulinas intravenosas en la medicina se remonta a hace más de 50 años, tras el uso como terapia sustitutiva en enfermedades inmunodeficientes. Sin embargo, las indicaciones de este tratamiento han evolucionado de tal manera que actualmente está dirigido a enfermedades donde el sistema inmune desempeña un papel relevante. En el campo de la dermatología se ha hecho un hueco interesante en algunas enfermedades, como la dermatomiositis, las enfermedades autoinmunes ampollares o la necrólisis epidérmica tóxica, entre otras. En los últimos años se ha prestado especial atención al uso de las inmunoglobulinas intravenosas, de hecho se han publicado recientemente nuevas guías sobre su uso, y qué consideraciones debemos tener en cuenta durante su uso en dermatología. Nuestra intención con este artículo es reflejar de una manera práctica el uso de las inmunoglobulinas intravenosas en la dermatología.
Immunoglobulins have been used for more than 50 years in the indication of primary and secondary immune system deficiencies. Their introduction in the treatment of different skin diseases is more recent, mainly because of the lack of randomized, controlled clinical trials and also because of the high costs. In 2008, the first guideline for the clinical use of intravenous immunoglobulin (IVIG) was published. Since then, publications have appeared in a wide range of journals describing their use for different conditions in isolated cases or in case series, culminating in recent guidelines. Between 3000 and 10 000 healthy donors are required to obtain an immunoglobulin concentrate. The production standards (World Health Organization 1982) were updated by the Committee for Propietary Medicinal Products of the European Medicines Agency (CPMP/BPWG/859/), with the aim of maintaining a higher level of quality and maximum safety in the manufacturing process. Plasma is incubated for at least 60 days to detect possible seroconversion of infectious agents (HBV, HCV, HIV, parvovirus B12, etc.). The functional integrity of the preparation is also tested for neutralizing antibodies and other immunomodulatory and inflammatory properties with the aim of detecting possible abnormalities in the function of these immunoglobulins. The national health agencies are responsible for regulating the manufacturing process as well as screening for viruses. Serum from donors positive for viral infections by polymerase chain reaction techniques or with abnormal immunoglobulin function is directly discarded to maintain the quality of the product.
Commercial IVIG preparations contain physiological quantities of all immunoglobulins except IgA, given that this immunoglobulin is responsible for anaphylactic reactions. The levels of this immunoglobulin should be kept as low as possible.
The bioavailability of IVIG is 100% at the time of infusion and 70% to 80% at 24hours. On the fifth day, 50% of administered product has been cleared. Although the half-life is estimated between 18 and 32 days, conditions such as fever or infections increase catabolism and, therefore, decrease the half-life. IVIG can pass through the placenta and is excreted in breast milk. In terms of mechanism of action, the Fc region of IgG can change signaling and transduction signs in cells that express Fcγ receptors on their surface, thus inducing both immune-mediated and anti-inflammatory changes. However, the mechanism of action of IVIG has not been fully clarified in in vivo studies. Recently, Pérez et al.1 published a very complete review of existing evidence of the use of IVIG in humans. The review aimed to provide the reader with a practical grounding in the use of this treatment in dermatology.
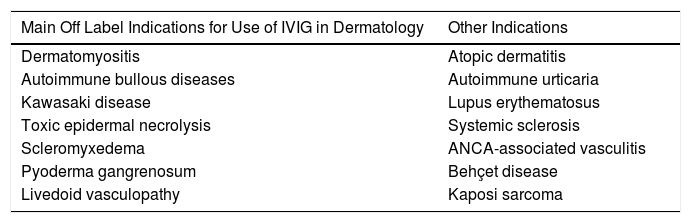
Main Indications for Intravenous Immunoglobulins in DermatologyAlthough the list of skin diseases in which IVIG therapy has been reported as a treatment option is quite extensive (Table 1), use was almost always off label. Nevertheless, good outcomes have been reported in some cases. In this article, we highlight the most relevant publications with the corresponding level of evidence and strength of recommendation (Table 1).
Main Skin Diseases for Which IVIG Can be of Use.
| Main Off Label Indications for Use of IVIG in Dermatology | Other Indications |
|---|---|
| Dermatomyositis | Atopic dermatitis |
| Autoimmune bullous diseases | Autoimmune urticaria |
| Kawasaki disease | Lupus erythematosus |
| Toxic epidermal necrolysis | Systemic sclerosis |
| Scleromyxedema | ANCA-associated vasculitis |
| Pyoderma gangrenosum | Behçet disease |
| Livedoid vasculopathy | Kaposi sarcoma |
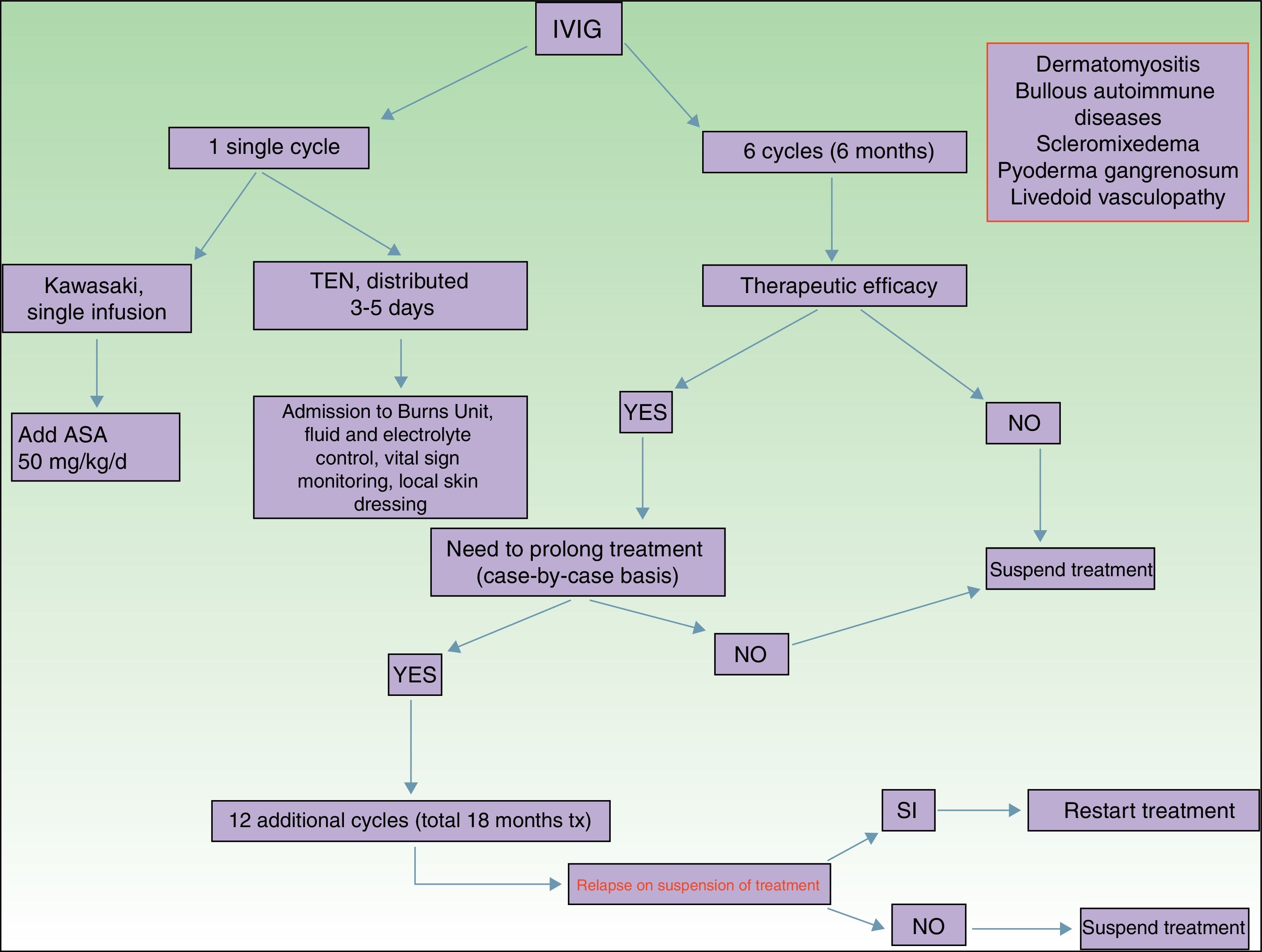
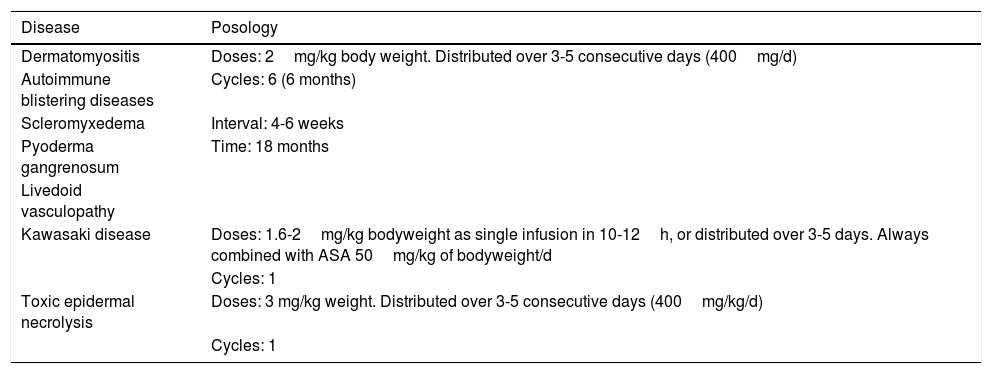
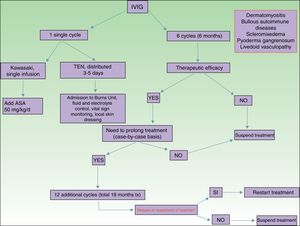
Of all the skin diseases described in this article, dermatomyositis, along with autoimmune blistering diseases, is perhaps the one with the highest level of evidence for efficacy. Placebo-controlled studies2 and multiple case series3 have been published, reporting satisfactory outcomes. IVIG is indicated as first-line therapy in cases of dermatomyositis with severe muscular involvement (fulminant myolysis), inclusion body myositis, and polymyositis.4 Cases of juvenile dermatomyositis,5 idiopathic dermatomyositis, and paraneoplastic dermatomyositis have been described with good response to treatment. IVIG is administered as adjuvant therapy, never as monotherapy, in patients who have not responded adequately to systemic corticosteroids after 1 month or in patients who experience worsening of muscle symptoms on decreasing the corticosteroid dose. The posology is described in Table 2. These agents are also useful in the treatment of skin manifestations associated with dermatomyositis, particularly when these are severe and extensive, even when muscular involvement is not present,6 or in the treatment of dystrophic calcinosis7 and calcinosis refractory to multiple immunosuppressants.8 Good outcomes have also been reported in severe edematous dermatomyositis9 and in dermatomyositis panniculitis.10 According to the systematic review by Callander et al.,11 IVIG therapy is an interesting alternative in the treatment of amyopathic dermatomyositis, as reported in isolated cases.12 In the treatment of juvenile dermatomyositis, IVIG therapy occupies a position as an effective and safe alternative,13 particularly when administered subcutaneously.14 This route of administration represents a major breakthrough in terms of safety and low rate of side effects, and it also reduces the number of school days missed.
Posology of IVIG Therapy by Dermatosis.
| Disease | Posology |
|---|---|
| Dermatomyositis | Doses: 2mg/kg body weight. Distributed over 3-5 consecutive days (400mg/d) |
| Autoimmune blistering diseases | Cycles: 6 (6 months) |
| Scleromyxedema | Interval: 4-6 weeks |
| Pyoderma gangrenosum | Time: 18 months |
| Livedoid vasculopathy | |
| Kawasaki disease | Doses: 1.6-2mg/kg bodyweight as single infusion in 10-12h, or distributed over 3-5 days. Always combined with ASA 50mg/kg of bodyweight/d |
| Cycles: 1 | |
| Toxic epidermal necrolysis | Doses: 3 mg/kg weight. Distributed over 3-5 consecutive days (400mg/kg/d) |
| Cycles: 1 |
Autoimmune blistering diseases are the second group of diseases in which IVIG therapy is an interesting treatment alternative,15,16 particularly in the severe forms and forms refractory to systemic glucocorticosteroids in combination with immunosuppressants (azathioprine and mofetil mycophenolate). IVIG therapy in the field of autoimmune blistering diseases is considered second-line treatment, although severe cases of pemphigus vulgaris, pemphigus foliaceus, mucous membrane pemphigoid,17 and acquired epidermolysis bullosa18 have been published with good therapeutic response. Other indications with a lower level of evidence for the use of IVIG therapy are bullous pemphigoid, linear IgA disease, IgA pemphigus, and paraneoplastic pemphigus. Table 2 shows the posology of IVIG therapy in blistering diseases. However, the use of rituximab in this group of diseases has been a great advance in the treatment and outcomes in these patients, with long disease-free periods.19 In patients with recalcitrant disease, the combination of rituximab and IVIG therapy has been shown to be effective,20,21 with complete and sustained responses; however, given the high cost of this combination, patients who would benefit from this therapeutic option should be selected appropriately. In our hospital, experience with the combination of rituximab and IVIG therapy, although limited to a small number of patients, has been satisfactory in those cases refractory to first-line drugs, as well as their possible combinations.
In certain special situations, such as when systemic glucocorticosteroids are contraindicated (aseptic bone necrosis, difficult-to-control diabetes mellitus, and severe osteoporosis), IVIG therapy could be justified as first-line treatment. However, this should always be as an adjuvant to other drugs such as immunosuppressants or rituximab, never as monotherapy. Good response to treatment can be assessed by the absence of new lesions and epithelization of existing ones, along with decreased autoantibody titers (IgA measured by enzyme-linked immunosorbent assay) or the possibility of reducing the dose of systemic glucocorticosteroids without disease deterioration.
IVIG therapy can also be indicated in gestational pemphigoid, a special situation given the therapeutic limitations associated with pregnancy, particularly when administration of glucocorticosteroids does not achieve sufficient disease control. The effectiveness of IVIG administration in cases of refractory gestational pemphigoid has been reported in several articles,22,23 in association with oral cyclosporin24 or as monotherapy,25 without any side effects for the neonate.
Kawasaki DiseaseLevel of Evidence i, Strength of Recommendation AAmong the vasculitis syndromes, Kawasaki disease is the only one in which IVIG is considered first-line therapy.26 Perhaps the most difficult aspect of this process is initial suspicion, as the patient may not meet all diagnostic criteria and therefore there is a delay in administration of treatment. The posology is described in Table 2. Some authors suggest IVIG administration as a single perfusion for 10-12hours, always in combination with acetylsalicylic acid. There are predictive factors for resistance to IVIG therapy,27 the most relevant currently being level of C-reactive protein (CRP) before starting treatment.28 The aim of treatment is to avoid formation of coronary aneurysms and associated comorbidities (ischemia, rhythm disorders, etc.). Decreased levels of CRP in plasma are the best indicator of good response to treatment.
Toxic Epidermal NecrolysisLevel of Evidence IIA, Strength of Recommendation BToxic epidermal necrolysis (TEN) is a severe toxicoderma with a mortality as high as 40% according to some published series. Apoptosis of keratinocytes is mediated by the action of Fas (CD95), granulysin, and annexin-1. Rapid identification of the process, withdrawal of the suspected causal drug, and admission to the intensive care unit for care of the patient as if they had severe burns are essential for improving the chance of survival. Several meta-analyses and systematic reviews have been published on the pharmacological treatment of TEN, given that major controversy exists as to the efficacy of administration of immunosuppressants during admission to hospital. The most recent clinical practice guidelines for TEN29 confer IVIG therapy an important role if administered early at high doses (3mg/kg), as a single cycle, split over 3 days at a slow infusion speed. It is essential to be aware of the patient¿s comorbidities, in particular renal insufficiency, cardiovascular diseases, and diabetes mellitus,30 as fractioning the dose over 3 to 5 days avoids decompensation due to fluid overload. The best sign for detecting suitable response to treatment is absence of epidermal detachment, as well as the start of re-epithelization of previously affected areas. Although numerous drugs have been used to treat this often fatal disease, basic skin and mucosal care, control of vital signs, and maintaining a good fluid and electrolyte balance seem to be the only interventions that have been shown to improve patient survival.31
ScleromyxedemaLevel of Evidence iii, Strength of Recommendation CScleromyxedema is a rare disease consisting of fibroblast proliferation and mucin deposition in the skin and internal organs, with resulting progressive hardening and fibrosis. It is usually a disease that is hard to treat, as it does not respond to multiple immunosuppressants and so is associated with high morbidity and mortality. In 2000, the first report appeared of good response to IVIG therapy.32 According to data published on patients with scleromyxedema with predominant neurocutaneous involvement, IVIG therapy is considered the treatment of choice, either alone or in combination with corticosteroids33 or plasmapheresis.34 The posology is described in Table 2. As with other dermatoses, factors predictive of good response to treatment correspond to skin changes, as well as improved function of internal organs.
Pyoderma GangrenosumLevel of Evidence iii, Strength of Recommendation CIVIG therapy can be considered as a treatment option in patients with severe pyoderma gangrenosum refractory to normal treatment (systemic glucocorticosteroids and oral cyclosporin A). The only reports correspond to single cases or small case series,35–37 all with severe pyoderma gangrenosum and good outcomes. It is recommended to administer IVIG therapy as an adjuvant to systemic glucocorticosteroids and/or cyclosporin A, although some cases of administration as monotherapy have been reported.
Livedoid VasculopathyLevel of Evidence iii, Strength of Recommendation DLivedoid vasculopathy presents with painful ulcers, above all on the legs, and these have a major impact on the patient¿s quality of life. The lesions can be refractory to multiple treatments. IVIG therapy has been used in severe difficult-to-treat cases with good response to treatment, according to published series.38–40 IVIG therapy is increasingly seen as a treatment option in this difficult-to-treat disease.
OthersThe scientific literature includes use of IVIG therapy in other diseases, with variable clinical response. However, the availability of new drugs with better response rates has displaced the use of IVIG therapy. These new options include in particular the use of dupilumab as an upcoming treatment of severe cases of atopic dermatitis41 and the use of omalizumab in autoimmune urticaria.42 Other diseases in which the role of IVIG therapy does not lead to similar therapeutic response to those described previously are systemic lupus erythematosus (level of evidence IIA, strength of recommendation C), particularly when associated with renal failure,43 systemic sclerosis44 (level of evidence I, strength of recommendation B), and the set of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated small-vessel vasculitis,45,46 such as granulomatosis with polyangiitis or eosinophilic granulomatosis with polyangiitis, and polyarteritis nodosa (PAN)-type vasculitis47 (level of evidence IIA, strength of recommendation B), where use of IVIG therapy is more questionable. In these cases, therapy is mainly reserved for those cases with a fulminant presentation that do not improve with systemic glucocorticosteroids in combination with cyclophosphamide.
Use of Intravenous ImmunoglobulinsGiven that there are several skin diseases that may benefit from IVIG therapy, it is necessary to be aware of certain characteristics summarized in the following points. For this review, we have referred to several articles related to the use of IVIG in dermatology. Of these, we would like to highlight 2 of these, the article by Pérez et al.1 and the European guidelines for the use of IVIG in dermatology,48 should the reader wish to go into more detail on the topic
PosologyThe dose used in all indications, except Kawasaki disease and TEN is 2g/kg body weight and cycle. The dose is distributed over 3 to 5 days, such that 400mg/kg body weight is administered daily. It is important to remember that dose adjustment is not needed for comorbidities, although it is recommended to control the volume of fluid administered in patients with renal or heart failure.
Rate of InfusionThe rate of infusion depends on the commercial preparation. An initial (first 30min) infusion rate of 0.8 drops/kg/min is recommended for preparations such as Flebogamma, with an increase to 1.6 drops/kg/min if well tolerated. For other preparations such as Privigen 10%, the initial rate of infusion is slower, 0.3 drops/kg/min, and this rate is increased if well tolerated after 30minutes, up to a maximum of 4.8drops/kg/min.
Monitoring Before and During AdministrationIt is essential to record a full medical history by organ and body system to detect a personal history of heart disease, chronic renal failure, diabetes mellitus, liver diseases, or administration of other drugs. It is recommended to assess kidney and liver function and measure blood glucose, determine hematology and coagulation parameters, as well as IgA, viral serology (HIV, HBV, HCV), cryoglobulins, blood group, and screen for thrombophilia. The patient should have signed the informed consent once treatment has been explained, along with the potential associated benefits and risks. It is important to keep the batch label, as this can help determine whether there is a problem with production in the event of infections or other treatment-associated complications.
Premedication and Grounds for Discontinuation Signs of toxicityPrior to IVIG infusion, it is recommended to intravenously administer 1 vial of Polaramine and paracetamol 1g. During treatment, blood pressure should be measured and the patient monitored for signs and symptoms that might induce suspicion of an adverse reaction, in particular, a sensation of chest oppression, rubeosis, hypotension, intense headache, or general progressive malaise. If any of these signs and symptoms appear, the rate of IVIG infusion should be reduced, and if they persist despite intravenous administration of Polaramine, corticosteroids, or even adrenaline, treatment should be suspended to due risk of anaphylactic shock.
In those patients at risk of pulmonary thromboembolism, 500mL of intravenous saline should be administered before and after IVIG infusion. Acetylsalicylic acid 100mg or heparin calcium 1000IU should be administered for 3 consecutive days after treatment.
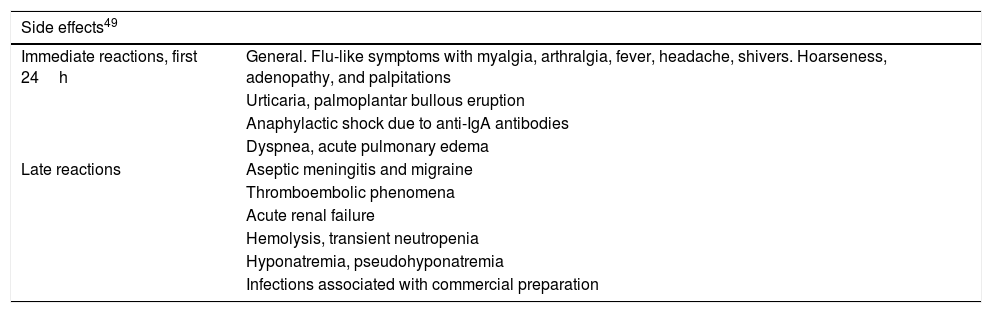
Precautions and ContraindicationsIn elderly patients or those with heart or kidney disease, hypercoagulability, systemic lupus erythematosus, migraine, and rheumatoid arthritis, particular care should be taken during administration of the treatment. With regards contraindications, severe hypersensitivity to IVIG therapy, severe kidney failure, and IgA deficiency are the most important. The US Food and Drug Administration considered IVIG as a pregnancy category C product. It is not contraindicated during breast feeding. Table 3 presents the side effects associated with administration of IVIG therapy.49
Main Side Effects of IVIG Therapy.
| Side effects49 | |
|---|---|
| Immediate reactions, first 24h | General. Flu-like symptoms with myalgia, arthralgia, fever, headache, shivers. Hoarseness, adenopathy, and palpitations |
| Urticaria, palmoplantar bullous eruption | |
| Anaphylactic shock due to anti-IgA antibodies | |
| Dyspnea, acute pulmonary edema | |
| Late reactions | Aseptic meningitis and migraine |
| Thromboembolic phenomena | |
| Acute renal failure | |
| Hemolysis, transient neutropenia | |
| Hyponatremia, pseudohyponatremia | |
| Infections associated with commercial preparation | |
There are many publications in the literature on the use of subcutaneous immunoglobulins as treatment for immunodeficiencies, myopathies, and other immune-mediated diseases.50–52 The use of this route of administration has opened up a new frontier in the use of immunoglobulins.53,54 Advantages include that no premedication is required, more physiological serum levels of IgG are obtained, and the patients’ quality of life is improved given they do not need to be admitted to hospital or attend a day hospital. Overall, this route of administration of immunoglobulins incurs lower health costs than IVIG. Infusion pumps are used to administer at a rate of between 15 and 25mL/h, distributing the total dose between different points of injection. Endovascular administration is contraindicated.
The indication of subcutaneous immunoglobulins, like IVIG, includes immunodeficiency conditions and autoimmune and inflammatory processes. Subcutaneous immunoglobulin administration can also be considered as maintenance treatment after initiation with IVIG. A maximum infusion rate of 20-25mL/h is recommended. Examples include the following preparations: Hizentra, Gammanorm, Subcuvia, Subgam, Gamunex-C, and Gammagard Liquid (Fig. 1).
ConclusionsUse of IVIG therapy in dermatology has become a treatment alternative in a range of diseases. Despite the limited number of controlled clinical trials, in publications on its use, good outcomes are obtained, with the advantage that this therapy is considered safe. It is necessary to be aware of the characteristics of administration, as well as associated side effects, use it appropriately, and avoid the appearance of unwanted side effects. Table 4.
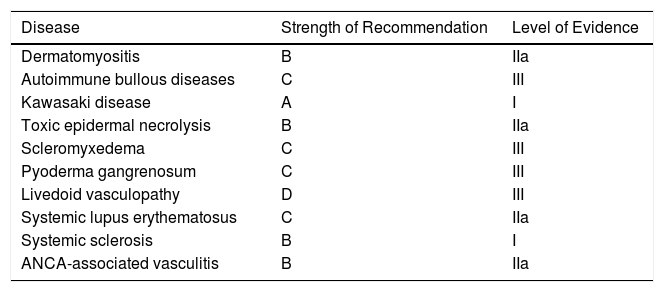
Strength of Recommendation and Level of Evidence for IVIG Therapy by Disease.
| Disease | Strength of Recommendation | Level of Evidence |
|---|---|---|
| Dermatomyositis | B | IIa |
| Autoimmune bullous diseases | C | III |
| Kawasaki disease | A | I |
| Toxic epidermal necrolysis | B | IIa |
| Scleromyxedema | C | III |
| Pyoderma gangrenosum | C | III |
| Livedoid vasculopathy | D | III |
| Systemic lupus erythematosus | C | IIa |
| Systemic sclerosis | B | I |
| ANCA-associated vasculitis | B | IIa |
The authors declare that they have no conflicts of interest.
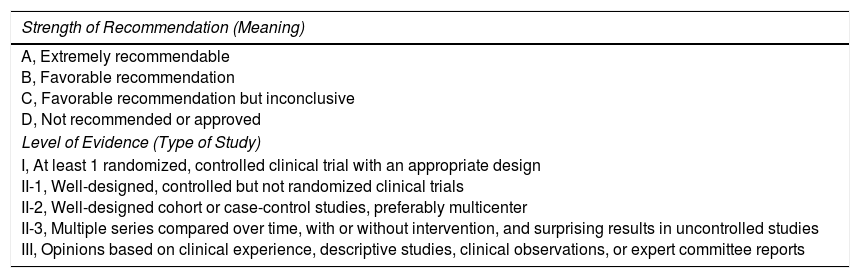
| Strength of Recommendation (Meaning) |
|---|
| A, Extremely recommendable B, Favorable recommendation C, Favorable recommendation but inconclusive D, Not recommended or approved |
| Level of Evidence (Type of Study) |
| I, At least 1 randomized, controlled clinical trial with an appropriate design II-1, Well-designed, controlled but not randomized clinical trials II-2, Well-designed cohort or case-control studies, preferably multicenter II-3, Multiple series compared over time, with or without intervention, and surprising results in uncontrolled studies III, Opinions based on clinical experience, descriptive studies, clinical observations, or expert committee reports |
Please cite this article as: Navarro-Triviño FJ, Pérez-López I, Ruíz-Villaverde R. Dermatología e inmunoglobulinas. ¿A quién y cómo administrarlas?. Actas Dermosifiliogr. 2018;109:323–330.