The basis for optimal resource allocation is an understanding of requirements during the diagnostic and treatment phases. Costs associated with the rising incidence of cutaneous melanoma are considerable. We undertook an up-to-date analysis of the cost of diagnosis, treatment, and follow-up according to tumor stage.
MethodsWe constructed descriptive tables following a theoretical model of direct costs based on amounts published in directives for the Spanish national health system and in international guidelines for managing cutaneous melanoma according to stage at diagnosis and clinical course. The tables allowed us to calculate the cost of treating individual patients as well as the expected cost for all patients with tumors in the same stage.
ResultsIndividual patients would generate costs ranging from €1689 (for a stage I tumor) to €88268 (stage IV). The largest differences were between stages IA and IB–IIA and between stages III and IV. Costs differed greatly between patients with early-stage tumors and favorable outcomes and those with recurring tumors, which cost 50-fold more in the first year and 20-fold more after 10 years of follow-up.
ConclusionsThe high cost of diagnosing advanced-stage cutaneous melanoma calls attention to the need to promote primary prevention and early detection. Our findings provide the knowledge base for cost-effectiveness studies in this disease.
El conocimiento de los recursos utilizados en cada uno de los pasos del diagnóstico y tratamiento de las enfermedades es la base para poder optimizarlos. El melanoma cutáneo es un tipo tumoral en constante incremento, y con un importante coste asociado, por lo que se ha realizado un análisis actualizado de los costes de los procesos de su diagnóstico, terapia y seguimiento en función del estadio de la enfermedad.
MétodosSe han elaborado tablas descriptivas de costes directos a partir de un modelo teórico basado en directrices nacionales e internacionales de manejo de pacientes con melanoma cutáneo dependiendo del momento de diagnóstico y evolución. Estas tablas permiten saber el coste de cada paciente individual y de todos aquellos en un mismo estadio.
ResultadosLos costes para un paciente en el primer año oscilan entre los 1.689 € del estadio I y los 88.268 € del estadio IV, las mayores diferencias se encuentran entre el estadio IA y el IB-IIA y entre el III y IV. Si comparamos los costes de los pacientes en estadio precoz con buena evolución con los de aquellos que recidivaron, las diferencias son considerables: llegan a ser de hasta 50 veces mayores en el primer año y 20 veces mayores en el seguimiento a 10 años.
ConclusionesLos elevados costes del diagnóstico del melanoma cutáneo en estadio avanzado evidencian la necesidad de promocionar la prevención primaria y los programas de detección precoz. Nuestros resultados servirán como base para posteriores estudios de coste-efectividad.
Cutaneous melanoma has become an important health problem in recent decades: an estimated 73870 new cases corresponding to a population incidence of 21.6 per 100000 inhabitants and 4.5% of all new cancer diagnoses occurred in the United States alone in 2015.1 Because there is no registry of all melanoma cases in Spain,2 we must extrapolate to calculate that about 10036 new melanoma diagnoses probably occur each year in this country.
The 2012 annual report on the Spanish National Health System reported a total expenditure of €98860 million in 2012, a figure that amounts to 9.3% of our gross domestic product. Population-adjusted health costs increased from €1978 per inhabitant in 2007 to €2095 in 2011, corresponding to an average annual increase of 1.4%.
The diagnosis and treatment of tumors accounts for nearly 10% of the national health system's spending. Ninety percent and 46% of this spending occurs in the last 6 and 2 months of patients’ lives, respectively.3
An article on US health expenditure described melanoma as one of the most costly cancers when diagnosis, treatment, and follow-up are all taken into consideration.4 A Spanish cost study concluded that the highest cost category was for metastatic melanoma and that prevention and awareness could contribute to large savings.3
Melanoma is a proliferation of atypical melanocytes in the epidermis. It can extend to the dermis and subcutaneous cellular tissue, evade the immune system5 while invading underlying tissues and metastasizing to other organs through the lymphatic or circulatory systems.6 This course of disease corresponds to the different stages in the classification system of the American Joint Committee on Cancer, in which stage I melanoma tumors are 1mm thick or less; stage II tumors measure between 1.01and 4mm and lymph nodes are unaffected; stage III has spread beyond the tumor to reach the lymph nodes; and stage IV involves distant metastasis.7
We aimed to assess costs related to melanoma in these 4 stages of disease, from the time of the first visit to the dermatologist until therapeutic and follow-up processes end. We identified points in diagnostic and treatment when the final costs generated by a patient could be modified.
Material and MethodsWe used the activity-based costing approach for this analysis, constructing tables showing costs for diagnostic, treatment, and follow-up activities recommended by clinical practice guidelines for treating patients with melanoma in each stage.
Amounts were extracted from official Spanish publications8–10 and adjusted to euro values for 2015 at an annual interest rate of 3%. The Official Gazette of the government of Navarre8 provided fees for hospital processes organized by diagnosis-related groups corresponding to the International Classification of Diseases, 9th Revision, Clinical Modification, and by procedures performed in an operating room. The only costs included that are not in the aforementioned categories corresponded to the anatomical therapeutic chemical classification system for active ingredients of drugs.3,9,10 Costs corresponding to human resources (hospital staff salaries) and maintenance of facilities are embedded in each concept. Costs incurred outside the hospital were excluded.
Our analysis was for care in keeping with Spanish clinical practice guidelines,11 but when such guidelines did not provide specific information, we used those of the National Comprehensive Cancer Network.12 Included were the cost of visits, diagnostic and related tests, surgery and medical treatments at each stage of the disease, with follow-up for 10 years. The frequency of follow-up activities varied by stage.
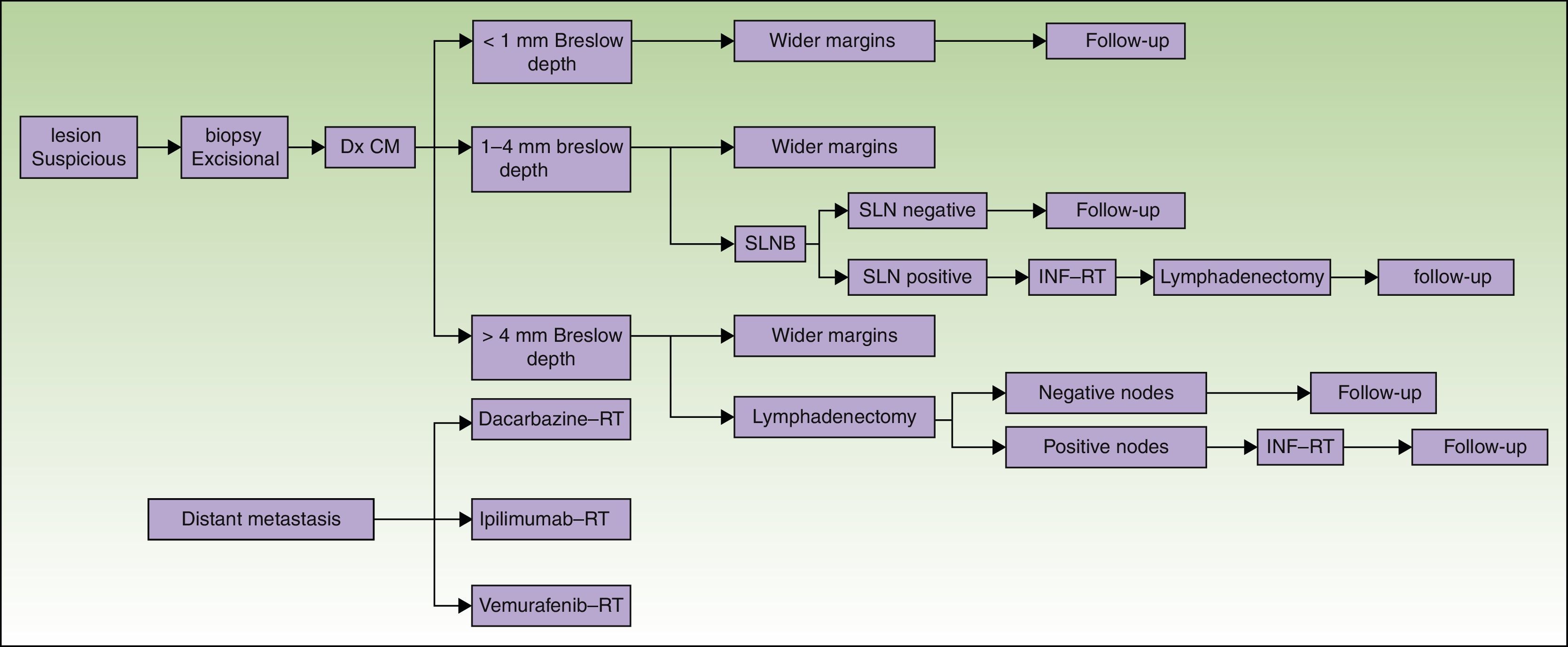
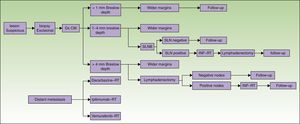
The states of health assessed correspond to the phases of the diagnostic or therapeutic processes involved (Fig. 1):
- 1.
Phase 1: Excision of the primary lesion. Applied to all patients, this step was the only treatment for tumors in stage I if no risk factors for recurrence were present (stage IA). For patients at low risk of recurrence excision was performed as minor surgery. Melanoma in situ was not included.
- 2.
Phase 2: Wide-margin surgery to excise the primary lesion and perform sentinel lymph node biopsy (SLNB) (stage II, in the absence of clinical signs of lymph node involvement).
- 3.
Phase 3: Metastasis to lymph nodes. If SLNB findings were initially positive or nodal disease was found on recurrence, nodes in the basin draining the tumor (stage III) were dissected. Further systemic or local treatment complemented the initial treatment.
- 4.
Phase 4: Systemic treatment in cases of distant metastasis (stage IV) at diagnosis or on recurrence.
Diagnosis and treatment protocol for melanoma. No consensus-based recommendation to perform SLNB when the Breslow thickness is between 1mm and 4mm is currently available. Therefore, SLNB was not included when calculating the cost of treating such tumors. Similarly, given that lymph node dissection for tumors with a Breslow thickness of more than 4mm is not performed routinely unless there are no palpable nodes confirmed by ultrasound for fine needle aspiration, the cost of dissection was not included. CM refers to cutaneous melanoma; Dx, diagnosis; INF, interferon; SLN, sentinel lymph node; SLNB, SLN biopsy; RT, radiotherapy.
The costs of wide-margin surgery with SLNB were weighted to reflect the complication rates with and without complete lymph node dissection13 (37.4% and 10.1%, respectively). In both cases costs were increased by 30% because 2 surgical procedures were performed during the same session.8 In addition, we also weighted SLNB costs by another 17% when multiple-nodal drainage basins were mapped, as in the Sunbelt Melanoma Trial.14
As part of the standard study of tumor extension we included laboratory analyses and CT scans if node involvement was detected. Such studies of extension are not recommended if the sentinel node is found to be negative for tumor cells.12 Positron emission tomography (PET) CT or magnetic resonance imaging could be ordered if the patient with advanced-stage melanoma was symptomatic, but these tests are not part of the standard protocol and were therefore not included in this cost study, with the sole exception of magnetic resonance imaging of the brain in stage IV melanoma.
When there was node involvement, radiation and interferon-α (INF) were prescribed and INF treatment was added to the final cost.11,12 Immune therapy with anti-CTLA-4 antibodies (1 dose every 3 weeks and a maximum of 4 treatments) was used for stage IV tumors, and these costs were also added.12 Other drug costs,10 such as for dacarbazine (2 cycles of 5 days every 21 days) and BRAF inhibitors (vemurafenib, 8 tablets/d for 3 months) were likewise included.10 Radiotherapy and radiosurgery could also be used in these cases, depending on type of metastasis and location. The costs of radiation for all patients were included.
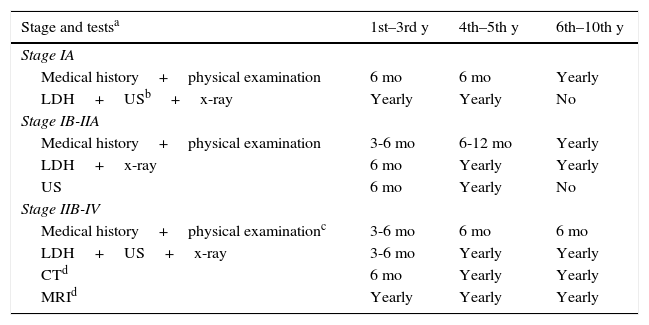
Follow-up schedules varied according to stage.11 Patients with stage IA melanoma attended 2follow-up visits in the first year, at which time lactate dehydrogenase levels were assessed and the nodal basin, abdomen and pelvis were explored by ultrasound. A chest radiograph was also ordered (Table 1). Patients with stage IB-IIA tumors were checked more often in the first and following years. Other tests included in the follow-up of stage IIB and higher varied according to year, patient symptoms, and risk factors. The cost of CT or PET-CT scanning was entered if those images were recommended in guidelines. Costs that contributed to better follow-up of disease were thus included.
Follow-up in Cutaneous Melanoma According to Stage.
| Stage and testsa | 1st–3rd y | 4th–5th y | 6th–10th y |
|---|---|---|---|
| Stage IA | |||
| Medical history+physical examination | 6 mo | 6 mo | Yearly |
| LDH+USb+x-ray | Yearly | Yearly | No |
| Stage IB-IIA | |||
| Medical history+physical examination | 3-6 mo | 6-12 mo | Yearly |
| LDH+x-ray | 6 mo | Yearly | Yearly |
| US | 6 mo | Yearly | No |
| Stage IIB-IV | |||
| Medical history+physical examinationc | 3-6 mo | 6 mo | 6 mo |
| LDH+US+x-ray | 3-6 mo | Yearly | Yearly |
| CTd | 6 mo | Yearly | Yearly |
| MRId | Yearly | Yearly | Yearly |
Abbreviations: LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; US, ultrasound.
CT and MRI according to recommendation 2A of the National Comprehensive Cancer Network, version 2.2015.12 Computed tomography or positron emission tomography–computed tomography. From 4 mo to 1 y, depending on patient risk factors.
Finally, total costs (for all diagnostic and treatment activities plus follow-up costs for 10 years) were analyzed according to stage at diagnosis.
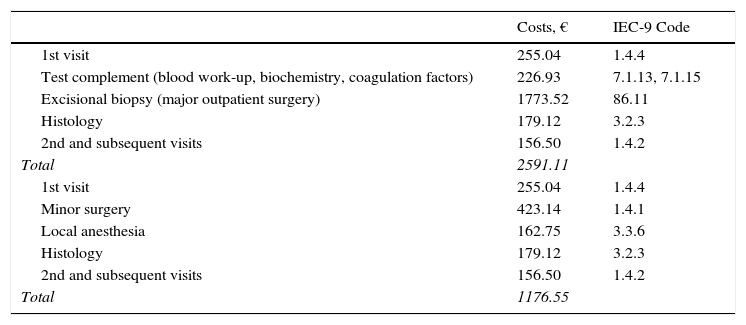
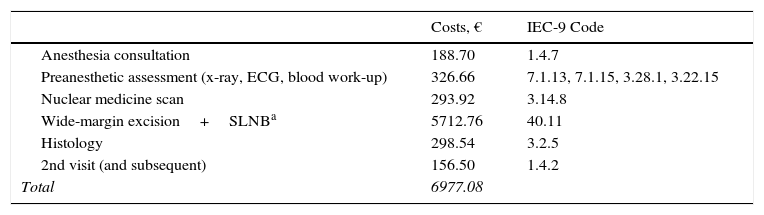
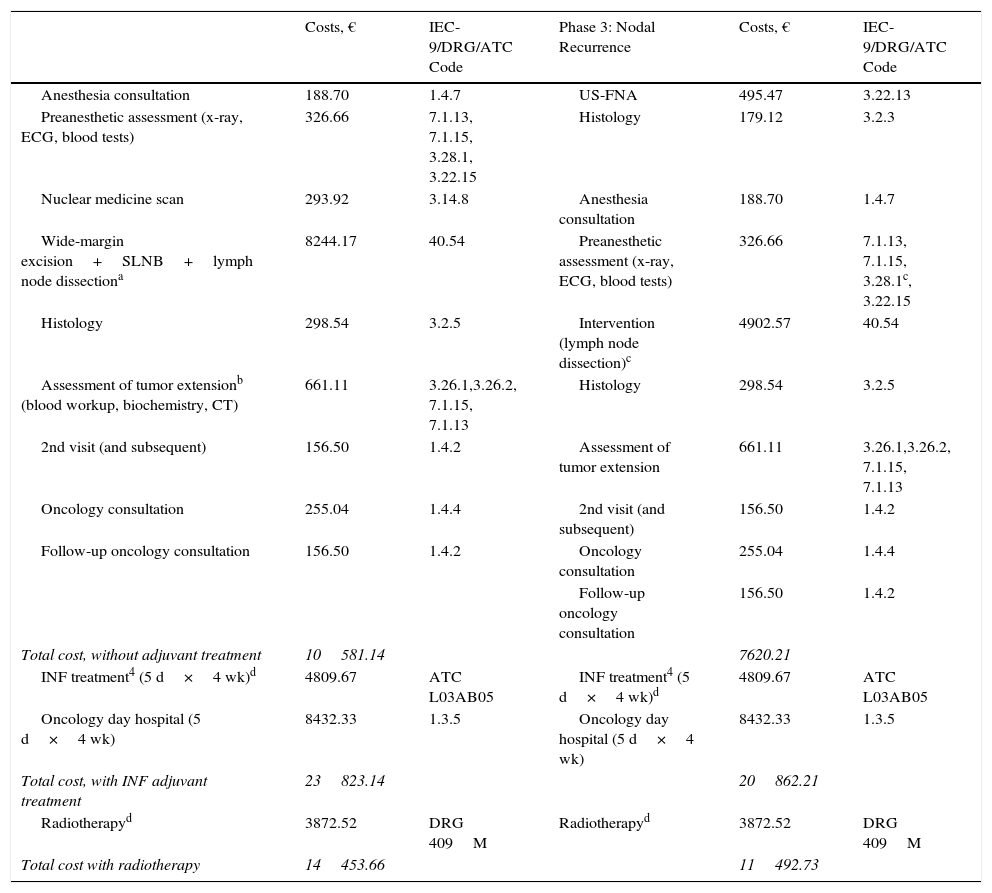
ResultsThe total cost of the diagnostic process and surgical treatment of a phase-1 primary lesion came to €2591 with a regimen of major outpatient surgery and €1177 with minor surgery (Table 2). The cost of wide-margin excision of primary melanoma tumors larger than 1mm plus SLNB are shown in Table 3. If the dissected lymph nodes were negative, the cost per patient was €6977. For cases with positive nodes, the cost with additional INF treatment (5-day cycles every 4 weeks) was €23823 (Table 4). If adjuvant radiation therapy was prescribed instead, the cost was €14454.
Costs of Diagnostic and Therapeutic Processes in Melanoma: 1st Phase—Excision of the Primary Lesion.
| Costs, € | IEC-9 Code | |
|---|---|---|
| 1st visit | 255.04 | 1.4.4 |
| Test complement (blood work-up, biochemistry, coagulation factors) | 226.93 | 7.1.13, 7.1.15 |
| Excisional biopsy (major outpatient surgery) | 1773.52 | 86.11 |
| Histology | 179.12 | 3.2.3 |
| 2nd and subsequent visits | 156.50 | 1.4.2 |
| Total | 2591.11 | |
| 1st visit | 255.04 | 1.4.4 |
| Minor surgery | 423.14 | 1.4.1 |
| Local anesthesia | 162.75 | 3.3.6 |
| Histology | 179.12 | 3.2.3 |
| 2nd and subsequent visits | 156.50 | 1.4.2 |
| Total | 1176.55 |
Costs of Diagnostic and Therapeutic Processes in Melanoma: 2nd Phase—SLNB With Negative Findings.
| Costs, € | IEC-9 Code | |
|---|---|---|
| Anesthesia consultation | 188.70 | 1.4.7 |
| Preanesthetic assessment (x-ray, ECG, blood work-up) | 326.66 | 7.1.13, 7.1.15, 3.28.1, 3.22.15 |
| Nuclear medicine scan | 293.92 | 3.14.8 |
| Wide-margin excision+SLNBa | 5712.76 | 40.11 |
| Histology | 298.54 | 3.2.5 |
| 2nd visit (and subsequent) | 156.50 | 1.4.2 |
| Total | 6977.08 |
Abbreviations: ECG, electrocardiogram; SLNB, sentinel lymph node biopsy.
Weighted cost of surgery with and without complications, considering a 10.1% complication rate: complication rate×cost of surgery with complications+(1–complication rate)×cost of uncomplicated surgery.15 Additional 30% when 2 surgical procedures were performed in a single operation.8 Seventeen percent of cases were assumed to involve multiple drainage pathways,14 incurring another 30% of added cost.8
Costs of Diagnostic and Therapeutic Processes in Melanoma: 3rd Phase—Spread to Lymph Nodes (Positive SLNB Findings).
| Costs, € | IEC-9/DRG/ATC Code | Phase 3: Nodal Recurrence | Costs, € | IEC-9/DRG/ATC Code | |
|---|---|---|---|---|---|
| Anesthesia consultation | 188.70 | 1.4.7 | US-FNA | 495.47 | 3.22.13 |
| Preanesthetic assessment (x-ray, ECG, blood tests) | 326.66 | 7.1.13, 7.1.15, 3.28.1, 3.22.15 | Histology | 179.12 | 3.2.3 |
| Nuclear medicine scan | 293.92 | 3.14.8 | Anesthesia consultation | 188.70 | 1.4.7 |
| Wide-margin excision+SLNB+lymph node dissectiona | 8244.17 | 40.54 | Preanesthetic assessment (x-ray, ECG, blood tests) | 326.66 | 7.1.13, 7.1.15, 3.28.1c, 3.22.15 |
| Histology | 298.54 | 3.2.5 | Intervention (lymph node dissection)c | 4902.57 | 40.54 |
| Assessment of tumor extensionb (blood workup, biochemistry, CT) | 661.11 | 3.26.1,3.26.2, 7.1.15, 7.1.13 | Histology | 298.54 | 3.2.5 |
| 2nd visit (and subsequent) | 156.50 | 1.4.2 | Assessment of tumor extension | 661.11 | 3.26.1,3.26.2, 7.1.15, 7.1.13 |
| Oncology consultation | 255.04 | 1.4.4 | 2nd visit (and subsequent) | 156.50 | 1.4.2 |
| Follow-up oncology consultation | 156.50 | 1.4.2 | Oncology consultation | 255.04 | 1.4.4 |
| Follow-up oncology consultation | 156.50 | 1.4.2 | |||
| Total cost, without adjuvant treatment | 10581.14 | 7620.21 | |||
| INF treatment4 (5 d×4 wk)d | 4809.67 | ATC L03AB05 | INF treatment4 (5 d×4 wk)d | 4809.67 | ATC L03AB05 |
| Oncology day hospital (5 d×4 wk) | 8432.33 | 1.3.5 | Oncology day hospital (5 d×4 wk) | 8432.33 | 1.3.5 |
| Total cost, with INF adjuvant treatment | 23823.14 | 20862.21 | |||
| Radiotherapyd | 3872.52 | DRG 409M | Radiotherapyd | 3872.52 | DRG 409M |
| Total cost with radiotherapy | 14453.66 | 11492.73 |
Abbreviations: ATC, Anatomical Therapeutic Chemical classification system; CT, computed tomography; DRG, diagnostic related group; ECG, electrocardiogram; INF, interferon; MRI, magnetic resonance imaging; RT, radiotherapy; SLNB, sentinel lymph node biopsy; US-FNA, ultrasound-guided fine-needle aspiration.
Weighted cost of surgery with and without complications, considering a 10.1% complication rate: complication rate×cost of surgery with complications+(1–complication rate)×cost of uncomplicated surgery.15 Additional 30% when 3 surgical procedures were performed in a single operation.8 Seventeen percent of cases were assumed to involve multiple drainage pathways,14 incurring another 30% of added cost.8
The assessment of tumor extension includes CT, PET, or MRI, depending on whether or not the patient is symptomatic. CT is not standardized. If SLNB findings are negative, assessment of extension is not recommended.12
Weighted cost of surgery with and without complications, considering a 10.1% complication rate: complication rate×cost of surgery with complications+(1–complication rate)×cost of uncomplicated surgery.15
Costs in cases of lymph node recurrence were similar, at €20862 with adjuvant INF and €11493 with adjuvant radiation (Table 4). The maximum cost for a patient with this clinical status was €30430 before follow-up, assuming an initially negative SLNB result.
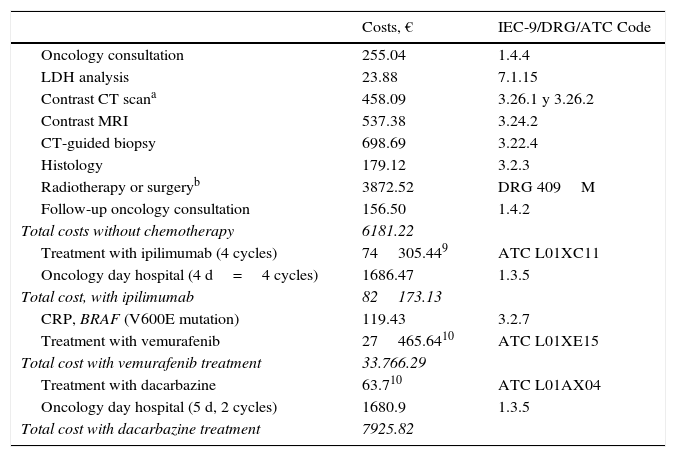
The costs generated for a patient with distant metastasis were those corresponding to excision of the primary tumor plus those for treating stage IV melanoma, giving totals of €84764, €36357, and €10517, depending on which chemotherapeutic drugs were used (Table 5). If distant metastasis were detected on recurrence of disease, the cost of SLNB was also added whether the biopsy results were positive (€108587) or negative (€91741). The cost of death was entered as zero.
Costs of Diagnostic and Therapeutic Processes in Melanoma: 4th Phase—Distant Metastasis.
| Costs, € | IEC-9/DRG/ATC Code | |
|---|---|---|
| Oncology consultation | 255.04 | 1.4.4 |
| LDH analysis | 23.88 | 7.1.15 |
| Contrast CT scana | 458.09 | 3.26.1 y 3.26.2 |
| Contrast MRI | 537.38 | 3.24.2 |
| CT-guided biopsy | 698.69 | 3.22.4 |
| Histology | 179.12 | 3.2.3 |
| Radiotherapy or surgeryb | 3872.52 | DRG 409M |
| Follow-up oncology consultation | 156.50 | 1.4.2 |
| Total costs without chemotherapy | 6181.22 | |
| Treatment with ipilimumab (4 cycles) | 74305.449 | ATC L01XC11 |
| Oncology day hospital (4 d=4 cycles) | 1686.47 | 1.3.5 |
| Total cost, with ipilimumab | 82173.13 | |
| CRP, BRAF (V600E mutation) | 119.43 | 3.2.7 |
| Treatment with vemurafenib | 27465.6410 | ATC L01XE15 |
| Total cost with vemurafenib treatment | 33.766.29 | |
| Treatment with dacarbazine | 63.710 | ATC L01AX04 |
| Oncology day hospital (5 d, 2 cycles) | 1680.9 | 1.3.5 |
| Total cost with dacarbazine treatment | 7925.82 |
Abbreviations: BRAF, B-Raf proto-oncogene; CRP, C-reactive protein; CT, computed tomography; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; PET, positron emission tomography; RT, radiotherapy.
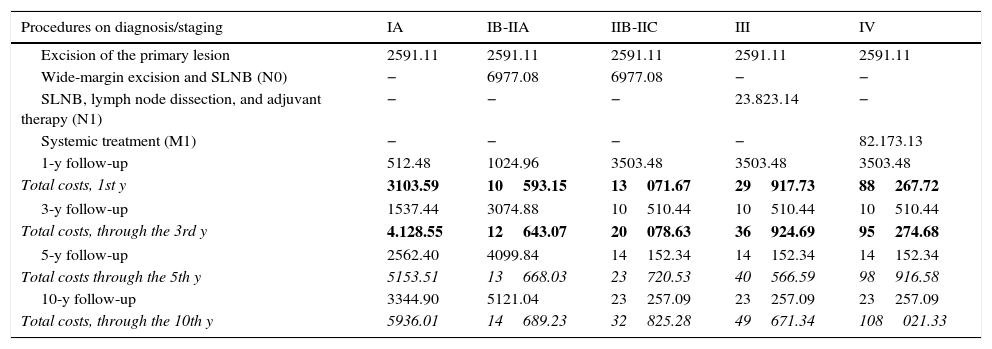
Follow-up costs varied according to stage and there were yearly variations for each stage11 as well; costs for the different time horizons are shown in Table 6 (maximum diagnostic costs). Thus, the costs generated for any patient can be calculated by adding, for example, the cost of INF treatment for a patient diagnosed with IIB-IIC cancer at high risk of recurrence.
Costs of Diagnostic, Therapeutic, and Follow-up Processes in Melanoma According to Stage.
| Procedures on diagnosis/staging | IA | IB-IIA | IIB-IIC | III | IV |
|---|---|---|---|---|---|
| Excision of the primary lesion | 2591.11 | 2591.11 | 2591.11 | 2591.11 | 2591.11 |
| Wide-margin excision and SLNB (N0) | − | 6977.08 | 6977.08 | − | − |
| SLNB, lymph node dissection, and adjuvant therapy (N1) | − | − | − | 23.823.14 | − |
| Systemic treatment (M1) | − | − | − | − | 82.173.13 |
| 1-y follow-up | 512.48 | 1024.96 | 3503.48 | 3503.48 | 3503.48 |
| Total costs, 1st y | 3103.59 | 10593.15 | 13071.67 | 29917.73 | 88267.72 |
| 3-y follow-up | 1537.44 | 3074.88 | 10510.44 | 10510.44 | 10510.44 |
| Total costs, through the 3rd y | 4.128.55 | 12643.07 | 20078.63 | 36924.69 | 95274.68 |
| 5-y follow-up | 2562.40 | 4099.84 | 14152.34 | 14152.34 | 14152.34 |
| Total costs through the 5th y | 5153.51 | 13668.03 | 23720.53 | 40566.59 | 98916.58 |
| 10-y follow-up | 3344.90 | 5121.04 | 23257.09 | 23257.09 | 23257.09 |
| Total costs, through the 10th y | 5936.01 | 14689.23 | 32825.28 | 49671.34 | 108021.33 |
Abbreviations: SLNB, sentinel lymph node biopsy.
The total costs for each stage within each time horizon (time frame) are shown in bold face.
To calculate the total cost for a patient with a tumor 1mm thick over a period of 8years after diagnosis, the cost of excising a phase-1 primary lesion must be added to phase-2 costs, if we are contemplating the most likely course of disease. That course would probably include a negative biopsy result (considering the rate of positivity is 15.9%).15 However, there would probably be recurrence by 3 years (as most recurrences appear within 2 to 3 years16). Phase-3 costs would thus be added to the costs for the first 3 years of follow-up for a stage IIA tumor and 5 years of follow-up for a stage III tumor. Thus the total cost would come to €47658. Should recurrence involve distant metastasis, the total cost of care would rise to €108969.
DiscussionThis cost analysis included the most recent data available for cutaneous melanoma in the Spanish national health system. We took into consideration the use of new technologies and therapies recommended by national and international guidelines, thus providing an up-to-date overview of the economic impact of melanoma over the natural course of this disease.
The premise underlying this study is that the first step in applying the tools of economic analysis to health care processes assumes that we are aware of actual costs. We therefore proposed a scenario encompassing all patients with a diagnosis of cutaneous melanoma and the possible variations in their clinical courses. Using this approach we were able to calculate the spending on health care resources for individual patients based on an understanding of costs generated in treating all patients diagnosed in the same stage of disease in Spain. Although the incidence of this disease is not among the highest reported, we estimate there are over 10000 new cases of melanoma that require resource allocation every year.
One of the most important findings of our study was the large rise in costs according to stage at diagnosis. Spending on treatment for patients with the worst prognosis can be up to 52-fold higher than that required by patients with the most favorable prognosis. The largest differences by stages were found between IA or IB-IIA and III or IV, in which costs are 6-fold and 3-fold higher, respectively. Another noteworthy finding was that costs more than doubled when there was lymph node involvement, consistent with a previous report.3 Follow-up costs were also found to differ by stage. First-year follow-up for patients in stage IA can be up to 7-fold higher than for patients already in stages IIB-IV. Yet another important finding was the small difference in the cost of treating lymph node involvement found during diagnosis versus during recurrence; the cost is higher when found during diagnosis.
Our results differed from those of an earlier study.3 We calculated costs for treating patients diagnosed in stages I and III to be twice as high as in the earlier study, mainly due to the current costs of the surgical procedure and possibly due to pricing differences in the Spanish autonomous communities studied. However, the greatest discrepancies between the 2 studies affected stages II and IV. Changes in treatments applied are mainly responsible for differences in stage IV costs.
What is evident in both studies is the clear rise in the cost of treating melanoma in different stages. Costs are particularly high in stage IV. Others have stressed the economic impact of early rather than late detection of cutaneous melanoma, pointing out the high direct costs of diagnosing the disease in advanced stages as well as the cost in time for patients, which also increases with stage because of more frequent follow-up testing and the fact that some treatments must be done in a hospital.4,17
Our findings show that higher spending in advanced stages is associated with differences in treatment approaches used. A standardized, effective treatment for metastatic melanoma is not currently available, even though this scenario calls for the avoidance of toxicity as well as conserving resources.18 The lack of cost-effective therapies in advanced melanoma argues in favor of preventing the disease or diagnosing it early. Australia, where the incidence of melanoma is among the highest in the world, has introduced prevention and awareness campaigns through which they diagnose more noninvasive melanomas, leading to considerable savings in health care costs.19
As mentioned, we have not included all possible treatment costs, given the large variety of individual or combined therapies. Nor have we considered the great variability among patients. Likewise, we have not calculated the impact of side effects, palliative care, or the treatment of local or in-transit recurrence. These limitations must be borne in mind. Our decision to assign a cost of zero to death is another limitation, given that some patients die in hospital or have used health care resources during their last year of life.
ConclusionsThis study has provided the basis for a cost-effectiveness analysis required for evaluating the techniques and therapies used to manage cutaneous melanoma and decide whether some activities might be replaced by others, potentially lowering costs without negatively affecting patient health. Future studies could usefully assess strategies for primary prevention of melanoma and early detection programs in the interest of reducing resource consumption, in fact, early diagnosis could lead to 50-fold lower costs in the first year. The fundamental goal would be to increase effectiveness as measured in gains in life-years or health quality-adjusted life years.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Serra-Arbeloa P, Rabines-Juárez ÁO, Álvarez-Ruiz MS, Guillén-Grima F. Estudio descriptivo de costes en melanoma cutáneo de diferentes estadios. Actas Dermosifiliogr. 2017;108:229–236.