Surgical excision is the treatment of choice for basal cell carcinoma (BCC). Complete excision with clear margins is important for reducing the risk of recurrence. The aims of this study were to describe the characteristics of BCCs in our health care area, calculate the percentage of positive margins after surgical excision, and determine the risk factors for incomplete excision.
Material and methodsRetrospective observational study of BCCs that were surgically removed at Hospital Universitario Nuestra Señora de Candelaria, in Santa Cruz de Tenerife, Spain, between January 1, 2014 and December 31, 2014. Information was collected on demographic, clinical, and histologic variables, surgical approach, margin status, and the department responsible.
ResultsIn total, 966 BCCs were diagnosed in 776 patients. Nine percent of tumors with complete data were biopsied, 89% were surgically excised, and 2% were removed by shave excision. The median age of patients with excised tumors was 71 years and 52% were men. BCCs were most often located on the face (59.1%). Surgical margins were analyzed in 506 cases, 17% of which had positive margins. Incomplete excision was significantly more common in tumors located on the face (22% vs. 10% for other locations) and in high-risk subtypes according to the World Health Organization classification (25% vs. 15% for low-risk subtypes).
ConclusionsThe characteristics of BCCs in our health care area are similar to those described elsewhere. Facial location and histologic subtype are risk factors for incomplete excision. Careful surgical planning is therefore important in the initial management of BCCs with these characteristics.
El tratamiento de elección inicial del carcinoma basocelular (CBC) es la escisión quirúrgica. Esta debería ser completa para reducir el riesgo de recidiva. Nuestro objetivo es conocer las características de los CBC en nuestra área de salud, el porcentaje de márgenes afectos y los factores de riesgo para una resección quirúrgica incompleta.
Material y métodosEstudio observacional retrospectivo de los CBC intervenidos en el Área de Salud del Hospital Universitario Nuestra Señora de Candelaria entre el 1 de enero de 2014 y el 31 de diciembre de 2014. Recogemos datos demográficos, clínicos e histológicos, servicio responsable, abordaje quirúrgico y estado de los márgenes.
ResultadosSe diagnosticaron 966 CBC correspondientes a 776 pacientes, siendo el 9% biopsias, el 89% escisiones y el 2% rebanados. La mediana de edad fue de 71 años y el 52% eran varones. La localización más frecuente fue la cara (59,1%). Se analizaron los márgenes quirúrgicos en 506 CBC. El 17% presentó afectación de márgenes. El porcentaje de márgenes afectos fue significativamente mayor en los tumores de la cara (22 cara vs. 10% otra localización) y en los de subtipo histológico de alto riesgo (OMS) (25 subtipo de alto riesgo vs. 15% bajo riesgo).
ConclusionesLas características de nuestros pacientes con CBC se asemejan a las descritas previamente. La localización facial y el subtipo histológico son factores de riesgo para la resección incompleta del CBC. Por lo tanto, el abordaje quirúrgico inicial de los CBC con estas características ha de planearse de forma cuidadosa.
Basal cell carcinoma (BCC) is the most common cancer and accounts for 75% of all skin cancers.1 The estimated lifetime risk of developing BCC in the white population is 30%.1 In addition, incidence is increasing in various countries, especially those closest to the equator, due to an aging population and sun exposure habits.1,2 The main carcinogenic factor involved in BCC is UV radiation, explaining why most tumors are located in sun-exposed areas.1 Surgery is the treatment of choice and is mostly curative, but some tumors have ill-defined borders or are located in surgically complicated areas. In such cases, incomplete excision is more likely. To our knowledge, no studies to date have analyzed the characteristics or management of BCCs or margin status following excision in the Canary Islands, a Spanish autonomous community with a subtropical climate and very intense daily sunlight (between 6 hours a day in winter and 11 hours a day in summer).3
The aims of this study were 1) to describe the epidemiological, clinical, and pathologic characteristics of BCC in the Southern Health Area of the province of Santa Cruz de Tenerife (ASSTF), 2) to analyze margin status following excision of BCCs by the dermatology department at Hospital Universitario Nuestra Señora de Candelaria (HUNSC), and 3) to identify risk factors associated with incomplete surgical excision.
Material and MethodsRetrospective observational study conducted in the health care area of HUNSC, a 960-bed tertiary-care hospital serving a population of approximately 500000 inhabitants. All patients diagnosed with BCC between January 1, 2014 and December 31, 2014 were included. They were identified by entering the terms carcinoma, skin, and biopsy into the PAT-Win pathology laboratory management software program. The database search was updated on March 14, 2022. The project was approved by the local ethics committee (code CHUNSC_2023_29).
Information was collected on clinical, epidemiologic, and histologic variables. Clinical and epidemiologic variables included sex (male/female); age at diagnosis (years); department responsible (dermatology, plastic surgery, maxillofacial surgery, ophthalmology, general surgery, otorhinolaryngology, unknown); number of BCCs diagnosed per patient; tumor location (nose, pinna, other parts of the face, scalp, neck, trunk, upper extremity, lower extremity); and tumor diameter. The tumors were classified into the following histologic subtypes: nodular/solid, infiltrative, superficial/multicentric, morpheaform/sclerodermiform, other. They were also classified as low risk (nodular, superficial, pigmented, infundibulocystic, or fibroepithelial) or high risk (basosquamous, morpheaform/sclerodermiform, infiltrative, or BCC with sarcomatoid or micronodular differentiation).4 We also analyzed the status of lateral and deep margins of tumors excised by the dermatology department. The search results were stored in an MS Excel (2019) spreadsheet, which was later exported to IBM SPSS version 19.0.
Statistical AnalysisThe characteristics of the specimens examined are described using nominal variables (frequency of component categories). Variables on a numerical scale are described using median and interquartile range (IQR). Nonnormal distribution was confirmed using histograms and the Kolmogorov–Smirnov test. Continuous variables were compared with the Mann–Whitney U test and nominal variables with the χ2 or Fisher exact test. Multivariate logistic regression analysis was applied using backward stepwise selection. The significance level set for the statistical tests used to process data (all bivariate) was P≤.05. The calculations were made in SPSS version 19.0.
ResultsWe identified 966 samples diagnosed as BCC in 776 patients. Surgical intent was not specified in 33 cases. Among the remaining 933 cases, there were 83 biopsies (9%), 827 excisions (89%), and 23 (2%) other techniques with curative intent (shave removal, shave removal and electrodessication). None of the patients were treated with Mohs micrographic surgery.
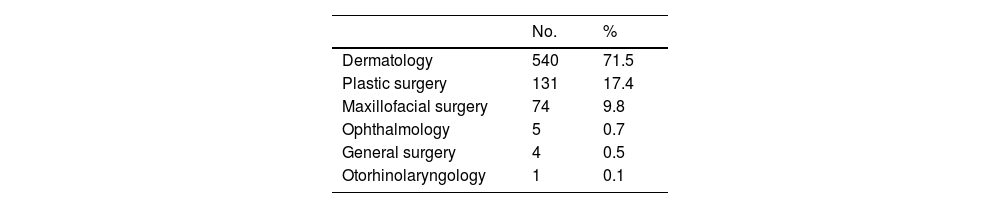
The 850 tumors treated with curative intent corresponded to 701 patients (362 men [51.6%] and 339 women [48.4%]). Median (IQR) age at diagnosis was 71 (60–73) years; no significant differences were observed between the sexes for age at diagnosis. A total of 149 patients (17.5%) were diagnosed with more than 1 BCC in the year analyzed, and the rate was significantly higher in men than women (20.8% [95] vs. 14% (54], χ2, P=.004). Most BCCs (71.5%) were removed by the dermatology department. The distribution of interventions by department is shown in Table 1.
Surgical Treatment of Basal Cell Carcinomas by Department.a
| No. | % | |
|---|---|---|
| Dermatology | 540 | 71.5 |
| Plastic surgery | 131 | 17.4 |
| Maxillofacial surgery | 74 | 9.8 |
| Ophthalmology | 5 | 0.7 |
| General surgery | 4 | 0.5 |
| Otorhinolaryngology | 1 | 0.1 |
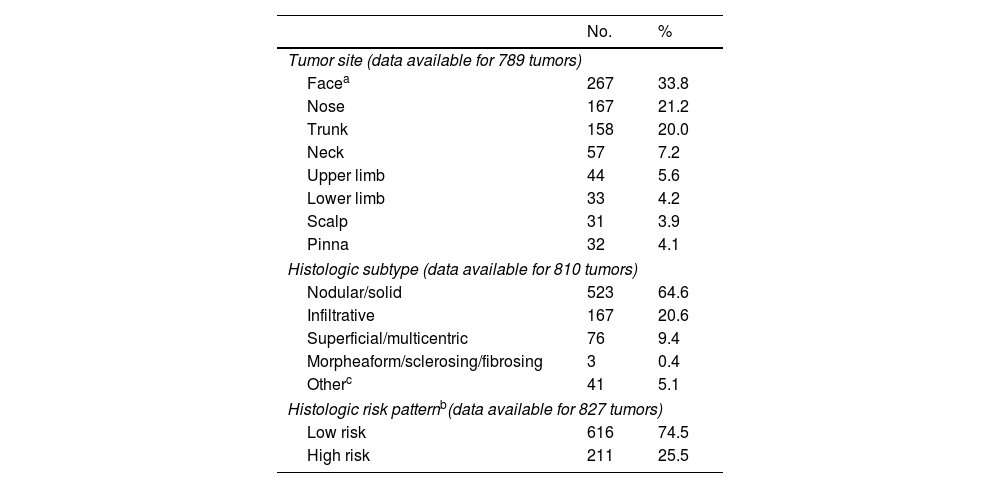
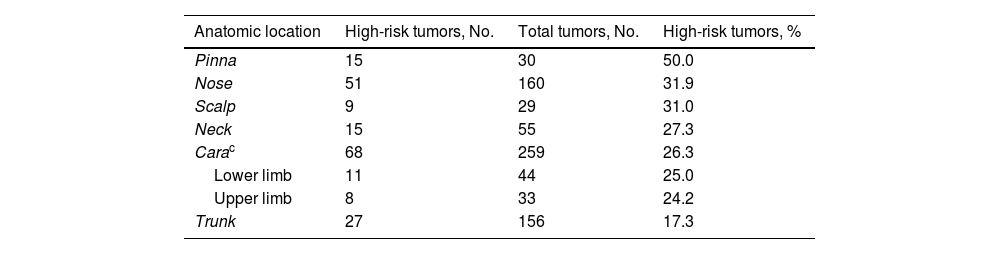
Tumor characteristics are summarized in Table 2. The most common location was the face (excluding the nose and pinna) (267 BCCs, 33.8%). The main histologic subtype was nodular (523, 64.6%); 211 tumors (25.5%) were classified as high risk and 616 (74.5%) as low risk. Proportionally, the pinna was the most common site for high-risk BCCs (50% of all cases) and the trunk the least (17.3%); 31.9% of tumors on the nose were high risk. These results are summarized in Table 3.
Main Characteristics of Basal Cell Carcinomas Analyzed.
| No. | % | |
|---|---|---|
| Tumor site (data available for 789 tumors) | ||
| Facea | 267 | 33.8 |
| Nose | 167 | 21.2 |
| Trunk | 158 | 20.0 |
| Neck | 57 | 7.2 |
| Upper limb | 44 | 5.6 |
| Lower limb | 33 | 4.2 |
| Scalp | 31 | 3.9 |
| Pinna | 32 | 4.1 |
| Histologic subtype (data available for 810 tumors) | ||
| Nodular/solid | 523 | 64.6 |
| Infiltrative | 167 | 20.6 |
| Superficial/multicentric | 76 | 9.4 |
| Morpheaform/sclerosing/fibrosing | 3 | 0.4 |
| Otherc | 41 | 5.1 |
| Histologic risk patternb(data available for 827 tumors) | ||
| Low risk | 616 | 74.5 |
| High risk | 211 | 25.5 |
| Anatomic location | High-risk tumors, No. | Total tumors, No. | High-risk tumors, % |
|---|---|---|---|
| Pinna | 15 | 30 | 50.0 |
| Nose | 51 | 160 | 31.9 |
| Scalp | 9 | 29 | 31.0 |
| Neck | 15 | 55 | 27.3 |
| Carac | 68 | 259 | 26.3 |
| Lower limb | 11 | 44 | 25.0 |
| Upper limb | 8 | 33 | 24.2 |
| Trunk | 27 | 156 | 17.3 |
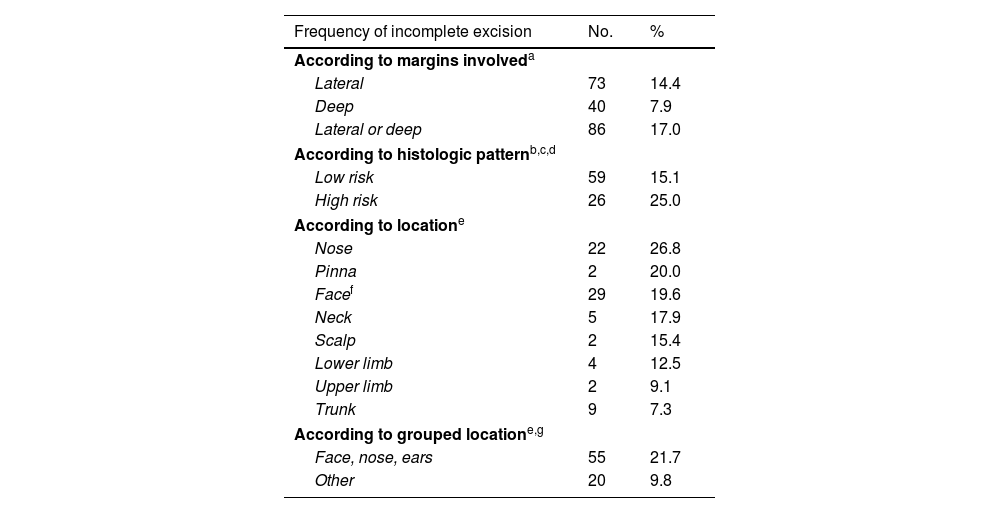
Overall, 517 of the 540 tumors treated with curative intent by the dermatology department were excised. Margin status was specified in 506 cases. The lateral margin was involved in 73 cases (14.4%), the deep margin in 40 (7.9%), and the lateral or deep margin in 86 (17%). In 37% of tumors with a positive lateral margin, the deep margin was also positive. It was also positive in 3% of tumors with a clear lateral margin (κ=0.418, P<.001).
Positive margins were observed in 26 tumors with a high-risk histologic pattern (25%) and in 59 of those with a low-risk histologic pattern (15.1%) (P= .017) (Table 4). BCCs with a high-risk subtype located on the nose and pinna were more likely to have involved margins (31.9% and 50%, respectively).
Analysis of Surgical Margins in Patients Who Underwent Surgical Treatment of Basal Cell Carcinomas in the Dermatology Department.
| Frequency of incomplete excision | No. | % |
|---|---|---|
| According to margins involveda | ||
| Lateral | 73 | 14.4 |
| Deep | 40 | 7.9 |
| Lateral or deep | 86 | 17.0 |
| According to histologic patternb,c,d | ||
| Low risk | 59 | 15.1 |
| High risk | 26 | 25.0 |
| According to locatione | ||
| Nose | 22 | 26.8 |
| Pinna | 2 | 20.0 |
| Facef | 29 | 19.6 |
| Neck | 5 | 17.9 |
| Scalp | 2 | 15.4 |
| Lower limb | 4 | 12.5 |
| Upper limb | 2 | 9.1 |
| Trunk | 9 | 7.3 |
| According to grouped locatione,g | ||
| Face, nose, ears | 55 | 21.7 |
| Other | 20 | 9.8 |
On grouping the tumors by location, most BCCs with positive surgical margins were located on the face (55, 21.7%), although 20 of these tumors (9.8%) were located in extrafacial areas (P<.001) (Table 3). Proportionally, most of the tumors with positive margins on the face were located on the nasal pyramid (26.8%, 22) followed by the auricle (20%, 2). The frequency of positive margins in other facial and extrafacial areas is shown in Table 4.
Multivariate logistic regression was performed with the 447 tumors for which information was available on sex, tumor, and histologic risk pattern. Both location and risk pattern were independent predictors of incomplete excision. The odds ratio (OR) for positive margins at risk sites (face, neck, nose, and pinna) was 2.51 (95% CI, 1.44–4.36; P=.001). The OR for positive margins in tumors with a high-risk histologic pattern was 1.77 (95% CI, 1.01–3.09; P=.044).
DiscussionA number of retrospective studies have analyzed the characteristics of surgically treated BCCs and the percentage of tumors with positive margins according to tumor location and histologic subtype. Although these studies have been conducted in a range of countries at different latitudes and with different UV indices (Australia, United States, United Kingdom, Netherlands, Switzerland, Italy, Turkey, Belgium, Spain), the results have been similar.5–22 The studies do, however, differ in design and the department publishing the results (mostly dermatology and plastic surgery departments). To our knowledge, this is the first study to describe the characteristics of BCCs and margin status following excision in the Spanish autonomous community of the Canary Islands.
The highest incidence rate for BCC has been reported in Australia (1541 cases per 100000 inhabitants a year23), followed by the United States and Europe.1,2,23–25 In Spain, crude incidence is estimated at 113.05 cases per 100000 person-years.24 It is plausible that the epidemiology of BCC in the Canary Islands differs to that of continental Spain and other regions in northern Europe, as it is a subtropical region with a much higher UV index.
Most BCCs develop in adults, particularly after the age of 50 years.1 The median age at diagnosis in the ASSTF was 71 years. Several studies have suggested that BCC is more common in men.25 Bassas et al.7 and Nagore et al.14 both reported male predominance (57.2% and 63%, respectively). In the present series, 51.6% of BCCs were diagnosed in men. Kumar et al.8 reported a rate of 50.2%. We found no significant differences between men and women for age at diagnosis in our series.
Coinciding with other series,7–10 most BCCs in the ASSTF (71.5%) were managed by the dermatology department (Table 1), with the treatment of choice being surgical excision.1 In the retrospective study by Bassas et al.,7 59.6% of BCCs were excised by the dermatology department, 21.5% by the plastic surgery department, and 15.5% by the general surgery department.
In our series, 17% of patients were diagnosed with more than 1 BCC during the year analyzed. Bassas et al.7 reported similar results, with 18% of patients presenting at least 2 BCCs. Kumar et al.8 reported a rate of 26.9%.
The face was the most common site for BCCs in our series (59.1%), and nasal tumors were particularly common (21.2%) (Table 2). The second most common site was the trunk (20%). Similar results have been described in the literature, with most BCCs located in sun-exposed areas, in particular the face6,8,9,11,13,14,16,18,19,23,26 and, within this area, the nose.5,12,14,20,23
The most common histologic subtype in our series was nodular BCC (64.6%) followed by infiltrative BCC (20.6%) (Table 2). These data coincide with previous reports.5,7,8,14–16,18,20 Emmet18 reported that 45% of BCCs were nodular, 35% multifocal, 8.9% morpheaform, and 8% infiltrative.
Reported rates of positive margins after surgical excision with curative intent range from 4% to 24%.5–7,9–15,18,20,21,26–28 In our series, 17% of tumors had involved margins, which were more common in tumors located on the face (29.6%) and tumors with a high-risk histologic pattern (25%) (Table 4). Nagore et al.14 found that BCCs with positive margins were more likely to be located on the face (20%–38% vs. 8% at other sites). Within the face, the rates were 38% for the nose and 35% for the perioral region. The authors also reported positive margins in 40% of morpheaform BCCs but in practically none of the superficial BCCs. Fleischer et al.10 found that a head and neck location was a significant risk factor for positive margins. Bozan et al.,11 by contrast, found no significant differences in margin positivity according to location or histologic subtype (P>.05).
European1 and American29 guidelines recommend using tumor size, location, and histologic subtype to predict the risk of positive margins in BCC. They also consider tumors located in the H area of the face (nasal area, pinnae, chin, temples) to be high risk. Other studies have described tumor size (>2cm) as a risk factor for incomplete excision.11,12,14,15,27 In our series, BCCs located on the face (29.6%), and more specifically on the pinnae or in the nasal area, had a greater risk of incomplete excision. High-risk subtypes were also more likely to be incompletely excised (25%) (Table 4), particularly when they were located at risk sites (31% incomplete excision rate for tumors with a high-risk growth pattern located in the nasal area and 50% for those located on the pinnae). Our findings coincide with those of Kappelin et al.,5 who reported higher incomplete excision rates for BCCs located on the face – especially in the nasal region (20.3%) and on the pinnae (23.7%) – and for aggressive histologic subtypes (morpheaform BCC [26.5%] and infiltrative BCC [7.5%]). The risk of positive margins was even higher for morpheaform tumors located on the nose or pinnae. Nonetheless, the overall incomplete excision rate (for all reported sites) was just 4.6%. One possible explanation for this lower rate may be related to tumor size, as complete resection would be more likely in tumors with a smaller diameter. Little, however, has been published on the influence of size on incomplete excision risk.5 One study that controlled for size found that a BCC diameter of 9mm or larger was a risk factor for incomplete primary surgical excision. Kumar et al.8 and Fleischer et al.,10 however, found no differences in this respect.
The level of agreement between lateral and deep margin involvement (42% in our series) is at the low end of what is considered moderate (41%–60%).30 Lateral margins have been more often involved in most of the studies published to date.7,8,12–15,27 Nagore et al.14 described a positivity rate of 21% for lateral margins and 15% for deep margins. The rates reported by Griffiths et al.12 were 5.3% for lateral margins, 2.3% for deep margins, and 0.8% for both lateral and deep margins.
Study LimitationsBecause of the retrospective design of this study, we were unable to analyze certain clinical variables that were not routinely recorded in the patients’ medical records (e.g., tumor diameter). We were also missing information on other variables, such as tumor site, which prevented us from analyzing tumors located in the H area of the face. One reason for missing information (e.g., details of treating department) may have been the adaptation period required following implementation of the hospital's new electronic medical system in 2014.
ConclusionsThe characteristics of patients with BCC in the ASSTF are similar to those described in other geographic locations. In line with previous reports, our findings show that facial location and, to a lesser extent, histologic subtype are risk factors for incomplete excision. Incomplete excision, mainly at risk sites, can have significant functional and cosmetic consequences.1 Careful surgical planning is therefore important for the initial management of BCCs with these characteristics.
Conflicts of InterestThe authors declare that they have no conflicts of interest.