Positive immunostaining for the tumor suppressor protein p16 is associated with the presence of mucosal or alfa subtypes of human papillomavirus (HPV) in cervical and genital squamous cell carcinoma (SCC). The aim of this study was to determine whether p16 immunostaining is also associated with mucosal HPV in extragenital SCC.
Material and methodsParaffin sections of lesions located in the genital region (8 genital warts, 3 intraepidermal SCCs, and 7 invasive SCCs) and extragenital area (29 intraepidermal SCCs corresponding to Bowen disease and 10 invasive SCCs) were stained for p16 by immunohistochemistry. Mucosal HPV was detected by polymerase chain reaction (PCR).
ResultsIn the genital area, p16 immunostaining was negative in genital warts and positive in all 3 intraepidermal SCCs and 2 invasive SCCs (29%). Mucosal HPV was detected in 6 genital warts and 2 intraepidermal SCCs (100% after exclusion of 3 lesions that could not be analyzed by PCR) and in the 2 invasive SCCs that were positive for p16. In the extragenital area, 19 intraepidermal SCCs (95%) and 2 invasive SCCs (20%) were immunopositive for p16. Mucosal HPV was detected in 4 intraepidermal SCCs (p16 immunopositive) and 1 invasive SCC (p16 immunonegative). In intraepidermal SCCs, p16 immunostaining facilitated the identification of dermal microinfiltration or invasion of normal skin appendages.
ConclusionsAccording to our results, unlike in genital SCCs, p16 immunopositivity is independent of the presence of HPV in extragenital SCCs. Compared with intraepidermal SCCs, the absence of p16 protein in invasive SCCs in the extragenital area would indicate progression of the disease.
La proteína p16 es una proteína supresora tumoral. El objetivo del estudio era comprobar si la tinción p16 se relaciona con la presencia de papilomavirus (subtipos mucosos o alfa, VPH-mc) en carcinomas epidermoides (CE) extragenitales (como ocurre en el cérvix y en CE genitales).
Material y métodoSe realizó tinción inmunohistoquímica con p16 a diversas lesiones incluidas en parafina del área genital (8 condilomas, tres CE intraepidérmicos y 7 CE invasores) y del área extragenital (20 CE intraepidérmicos tipo enfermedad de Bowen [EB] y 10 CE invasores). La detección de VPH-mc se realizó mediante reacción en cadena de la polimerasa (PCR).
ResultadosEn el área genital la tinción p16 fue negativa en los condilomas y positiva en los tres CE intraepidérmicos y en dos CE invasores (29%). Se detectó VPH-mc en 6 condilomas y dos CE intraepidérmicos (100%, excluyendo tres lesiones que no se pudieron estudiar con PCR) y en los dos CE invasores positivos para p16. En el área extragenital la tinción p16 fue positiva en 19 EB (95%) y en dos CE invasores (20%). Se detectó VPH-mc en 4 EB (tinción p16 positiva) y en un CE invasor (p16 negativa). En los CE intraepidérmicos la tinción p16 fue útil para objetivar si existían focos de microinfiltración dérmica o invasión de estructuras anexiales normales.
ConclusionesSegún nuestros resultados la positividad de p16 es independiente de la detección de VPH en los CE extragenitales, al contrario de lo observado en CE genitales. En el área extragenital la pérdida de proteína p16 en los CE invasores respecto a los CE intraepidérmicos indicaría progresión tumoral.
The retinoblastoma gene (RB1) regulates the G1/S transition in the cell cycle. When the RB1 protein is dysfunctional the cell cycle can progress even in the presence of unrepaired DNA abnormalities.1–3 Chromosome 9p21 (locus CDKN2A) encodes 2 tumor suppressor proteins, p16INK4a and p14ARF. p16INK4a (p16) inhibits cyclin-D1-dependent kinases 4 and 6 (CDK4 and 6), whose function is to regulate RB1 activity through phosphorylation. Loss of p16 function permits deactivation of RB1 through hyperphosphorylation, leading to dysregulation and premature progression of the cell cycle.1–3
Abnormalities in p16 have been reported to be inherited (as occurs in familial predisposition to melanoma)2 following spontaneous mutations or induced by UV exposure4,5 or the action of human papillomavirus (HPV)5–8: mucosal (α) HPV types (including HPV 6, 11, 16, 18, 31, 33, and 35) act on the RB1 gene, leading to overexpression of p16. Specifically, protein E7 of these HPV types binds to RB1 and impedes access of transcription factors to the gene.6,9–11
In uterine cervical carcinomas and their precursor lesions, positive p16 immunostaining has been associated with highly oncogenic mucosal or α HPV types, that is, HPV types 16, 18, 33, 35 among others. A similar pattern arises in epidermal carcinomas of the genital area, such as carcinomas of the vulva, penis, and perineum.6–9
In the extragenital region, p16 staining is positive in 80% to 90% of the intraepidermal squamous cell carcinomas (SCCs) of Bowen disease12–15; positivity has also been reported with staining for p16 (in lower percentages) in actinic keratoses and in invasive SCCs, although some studies have reported very variable and even contradictory results.1,2,15–18
We aimed to investigate whether p16 positivity in extragenital lesions was associated with the presence of oncogenic mucosal HPV, as is the case in uterine cervical carcinomas and SCCs of the genital area.6–8 Although similar studies had been performed, this was the first in Spanish patients. We would thus be in a position to assess whether the Spanish population and previously studied populations differed from one another. We studied different lesions of the genital area in which mucosal HPV is implicated in pathogenesis, such as genital warts and SCCs of the vulva and penis,6,7 and SCCs of the extragenital area in which pathogenic involvement of mucosal HPV types is open to debate, as is the case with the intraepidermal SCCs of Bowen disease,12–15 or nonexistent, as is the case with actinic keratosis and invasive SCC (epidermodysplasia-verruciformis-associated or β HPV types but not mucosal or α HPV types have been implicated in the pathogenesis of these lesions10,11).
Materials and MethodsAll histology samples were obtained from the pathology tissue bank at Hospital Universitario Virgen de la Arrixaca in Murcia, Spain; all samples had been embedded in paraffin. A descriptive, observational study was undertaken of p16 immunostaining in 48 lesions.
- 1.
Genital lesions: 8 genital warts and 10 SCCs of the vulva or penis.
- 2.
Extragenital lesions: 20 intraepidermal Bowen-disease–type SCCs (8 located on the head, 2 on the arms, 6 on the legs, and 4 on the trunk) and 10 invasive SCCs, all located on the head.
All lesions were reexamined to confirm the original diagnosis. Subsequently, immunohistochemical staining with p16 was undertaken using the immunoperoxidase technique (anti-p16 antibodies, mtm laboratories AG, CINtec Histology Kit, Clone E6H4). Staining for p16 was considered positive when the cell nuclei were stained, regardless of cytoplasmic staining. Diffuse positivity was defined as when p16 staining was positive in more than 50% of cells or in all cells of different epidermal layers, focal positivity as when staining occurred in more than 10% of isolated cells, and negativity when no staining was observed or only occurred in less than 10% of isolated cells.
As a control for p16 immunostaining, immunoperoxidase was used without anti-p16 antibodies; eccrine glands and cells from the matrix of the sebaceous glands and normal hair follicles were also used as these are normally positive for p16 staining.
Statistical AnalysisThe data were processed using the SPSS 12.0 statistical package for Windows.
The results obtained were analyzed by producing contingency tables with the Pearson χ2 test, considering a probability of error below 5% as significant (P<.05). When few samples were available, the Fisher exact test was used.
Study of Human Papillomavirus by Polymerase Chain ReactionThe paraffin blocks under study were cut into 5 4-μm sections using a new scalpel for each sample. DNA was extracted using the Maxwell 16 automated system (Promega, Madison, USA) and the Case Work DNA extraction kit (Promega, Madison, USA); 100 ng/L of DNA was used for 2 polymerase chain reaction (PCR) amplifications: 1 using GP5+/6+primers and the other using the short PCR fragment (SPF) system. A fragment of the human TBXAS1 gene was used as an endogenous control.19
Samples were considered as negative for HPV when no PCR product was obtained in any of the amplification steps in which GP5+/6+ and SPF primers were used and amplification of the human TBXAS1 gene was positive. Samples amplified with GP5+/6+ and/or SPF were typed with the Hybridio HPV GenoArray typing kit (Hybribio, Hong Kong, China). This kit detects the following HPV genotypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 (high risk genotypes); 6, 11, 42, 43, and 44 (low risk genotypes); and 53 and CP8340 (intermediate risk genotypes). The samples that were negative for these types were sequenced using the Abi Prism 3130 Genetic Analyzer (Applied Biosystems, USA).
ResultsThe main results are presented in Tables 1 and 2.
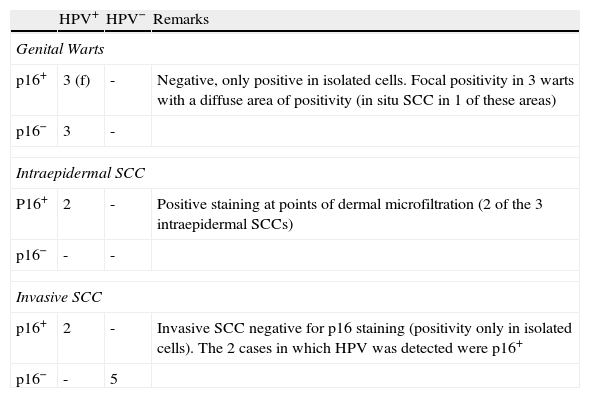
p16 Staining in Genital Lesions.
| HPV+ | HPV− | Remarks | |
| Genital Warts | |||
| p16+ | 3 (f) | - | Negative, only positive in isolated cells. Focal positivity in 3 warts with a diffuse area of positivity (in situ SCC in 1 of these areas) |
| p16− | 3 | - | |
| Intraepidermal SCC | |||
| P16+ | 2 | - | Positive staining at points of dermal microfiltration (2 of the 3 intraepidermal SCCs) |
| p16− | - | - | |
| Invasive SCC | |||
| p16+ | 2 | - | Invasive SCC negative for p16 staining (positivity only in isolated cells). The 2 cases in which HPV was detected were p16+ |
| p16− | - | 5 | |
Abbreviations: f, focal positivity; HPV+, human papillomavirus detected by polymerase chain reaction; SCC, squamous cell carcinoma.
Staining for p16 in Extragenital Squamous Cell Carcinomas.
| HPV+, % | HPV−, % | Remarks | |
| Bowen disease | |||
| p16+ | 4 | 15 | Diffuse and intense positivity in all epidermal layers of BD. |
| p16− | - | 1 | Four cases with HPV (predominance of HPV-16) |
| Invasive SCC | |||
| p16+ | - | 2 | In the 8 p16-negative cases, focal positivity in the epidermis adjacent to the invasive SCC (areas of dysplasia and/or AK). One case with HPV-16 |
| p16− | 1 | 7 | |
Abbreviations: AK, actinic keratosis; BD, Bowen disease; HPV, human papillomavirus; PCR, polymerase chain reaction; SCC, squamous cell carcinoma.
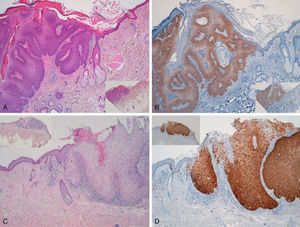
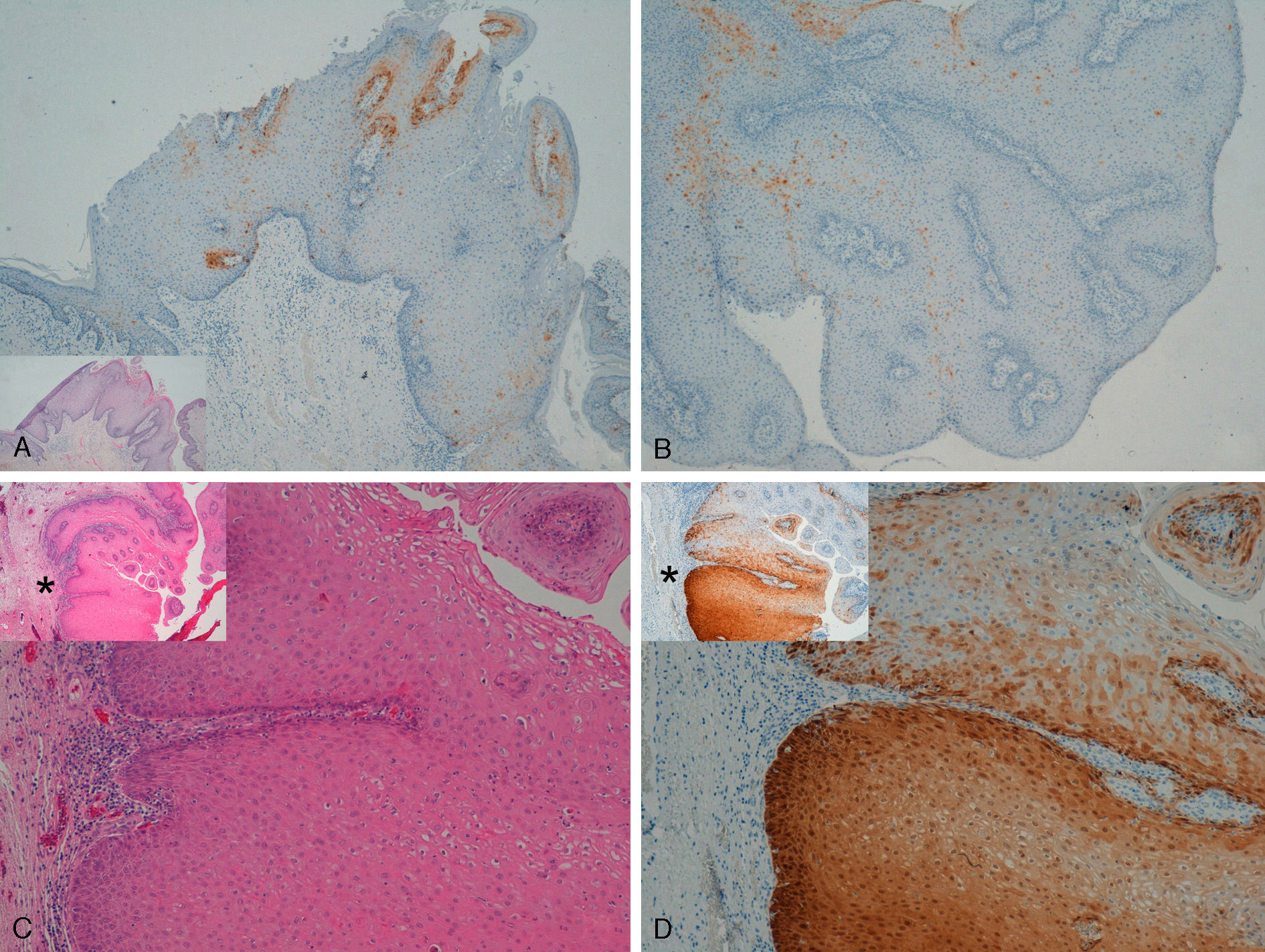
Immunohistochemical staining for p16 was negative in 5 of the 8 genital warts (positivity was only observed in isolated cells, and in fewer than 10%) (Fig. 1A and B). In the other 3 genital warts, we observed focal positivity (in isolated cells and more intense and diffuse positivity in an area of the genital wart) (Fig. 1C and D); in 1 of those areas with strongest positivity there was evidence of severe dysplasia or in situ SCC (Fig. 2).
A and B, Negative staining for p16 in genital warts (positivity only in isolated cells). A, p16 staining (original magnification x16); inset shows hematoxylin and eosin (original magnification x16). B, p16 staining (original magnification x40). C and D, Genital wart of the vulva with a positive area for p16, without histologic evidence of carcinoma. C, Hematoxylin and eosin staining (original magnification x100). D, p16 staining (original magnification x100). Insets in C and D show lower-magnification views (original magnification x40).
Two genital warts had abnormal or degraded DNA, that is, an “inhibited sample,” and so it was not possible to determine whether HPV was present or not. The other 6 genital warts could be analyzed and low-risk mucosal HPV was detected in all of them (4 cases of HPV-6 and 2 of HPV-11). In the 3 samples with a larger area of positivity for p16 (focal positivity), HPV-6 was detected in 2. HPV-11 was detected in the sample that showed evidence of in situ SCC. In the remaining genital warts, which were p16 negative, HPV-6 was detected in 2 samples and HPV-11 in 1 sample, while 2 samples had degraded DNA.
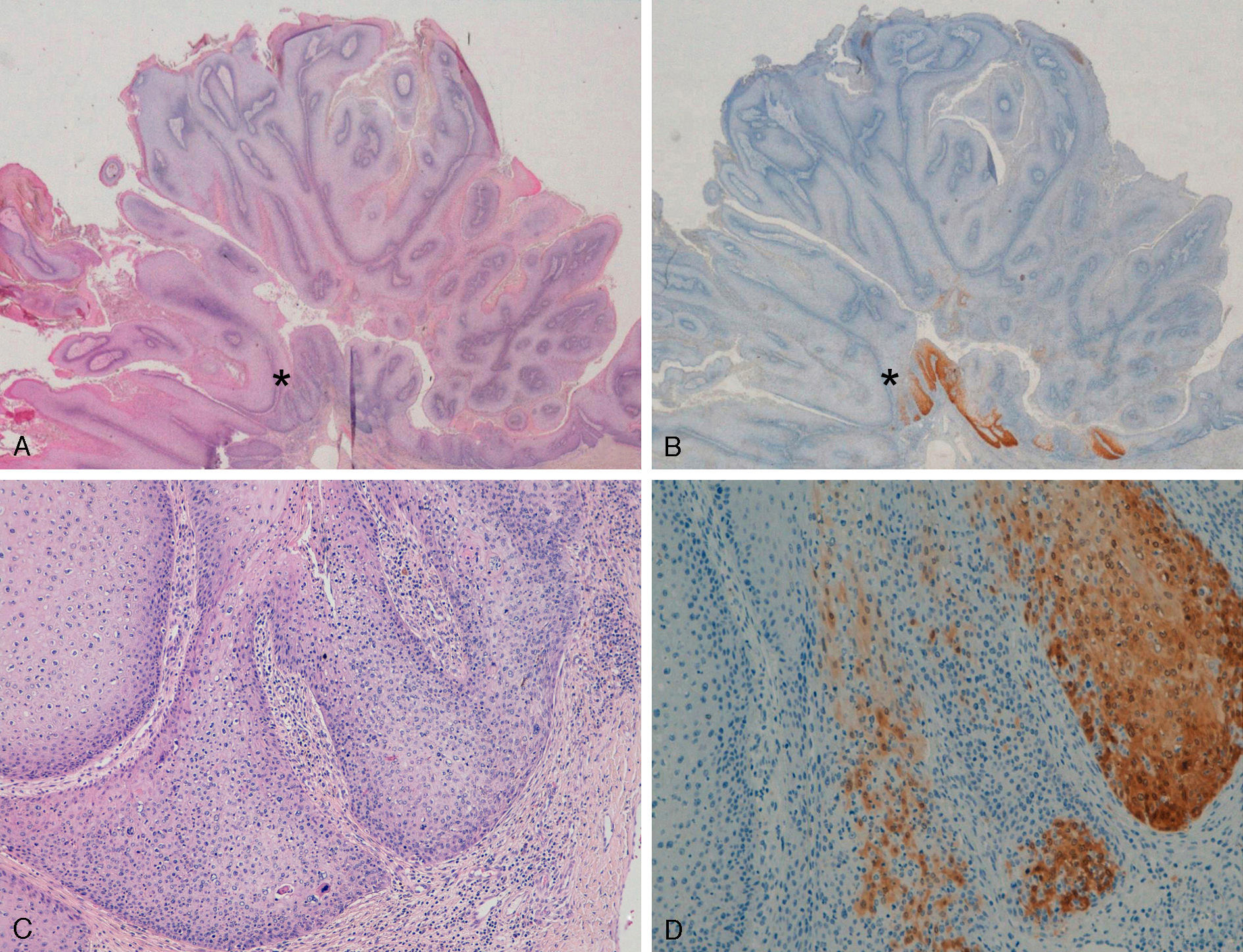
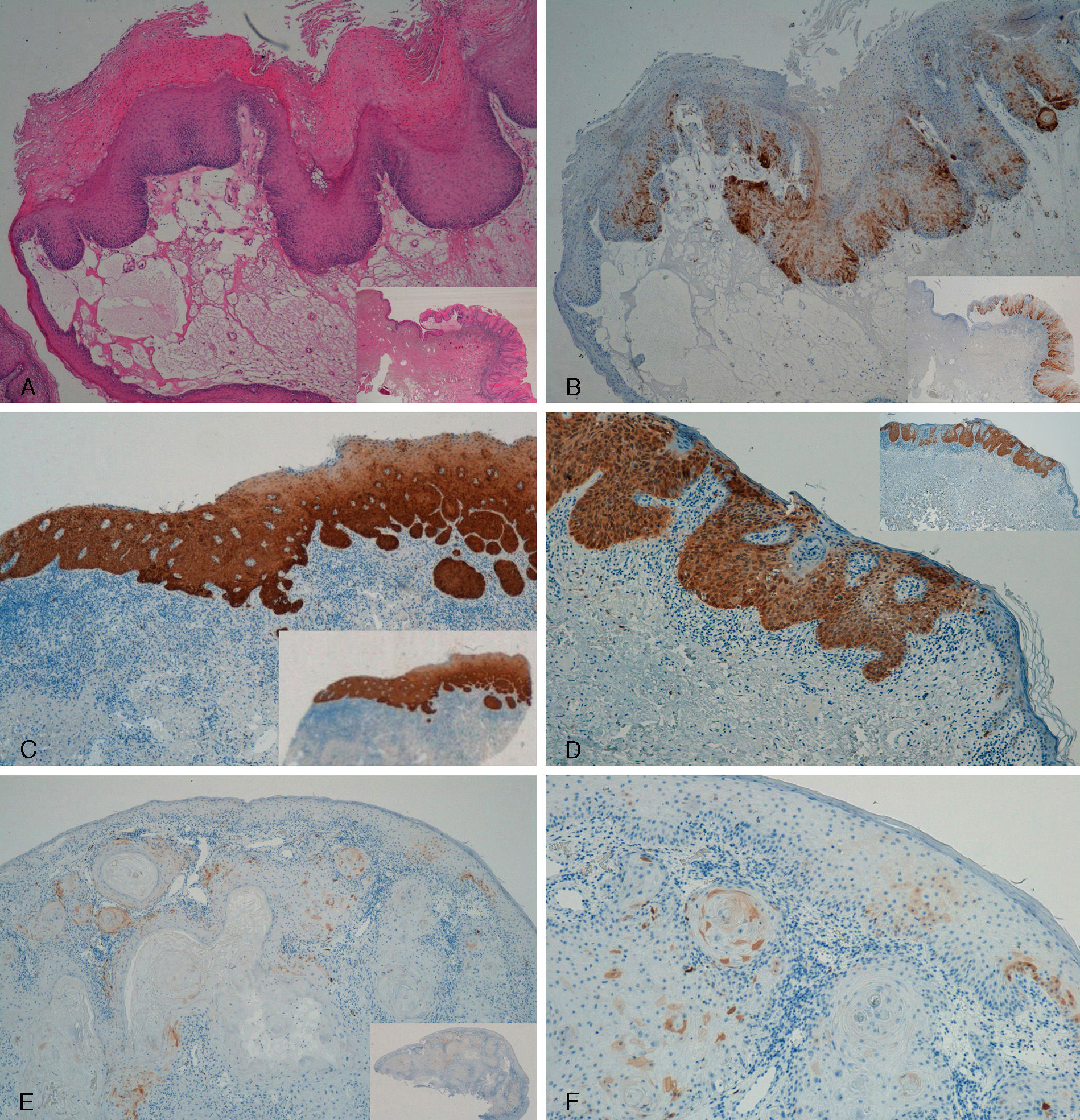
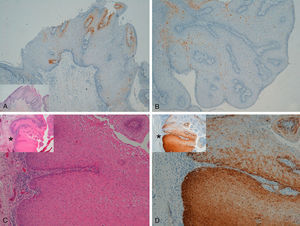
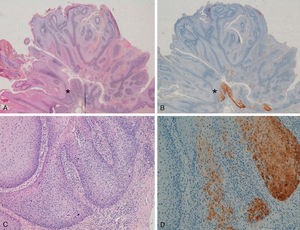
With regard to the 10 SCCs, 3 were considered intraepidermal and were positive for p16 (Fig. 3A-3D); HPV-33 and HPV-6 were detected in 2 of these, whereas in the third the DNA had been degraded. In 2 of these intraepidermal SCCs, a small region of microinfiltration was observed in the dermis and this was also positive for p16 (Fig. 3C).
A and B, Intraepidermal carcinoma of the penis with positive staining for p16 in the lower third of the epidermis (A, hematoxylin and eosin staining [original magnification x40]; B, p16 staining [original magnification x40]; insets show lower-magnification views [x16]). C, Intraepidermal carcinoma of the vulva, positive for p16 throughout the epidermis, with some points of microinfiltration in the dermis (original magnification x40, inset shows lower-magnification view [x16]). D, Intraepidermal carcinoma of the penis. Positive staining for p16 throughout the epidermis (original magnification x40, inset shows lower-magnification view [x16]). E and F, Invasive squamous cell carcinoma of the vulva, with p16 staining negative in the tumor (original magnification x40 in E and x100 in F; inset in E shows lower-magnification view [x16]).
The other 7 SCCs were invasive, 5 were negative for p16, with positivity only in isolated cells and in fewer than 10% overall (Fig. 3E and 3F). The other 2 were positive for p16. High-risk mucosal HPV—HPV-33 and HPV-16—was detected in 2 samples, both positive for p16. In the other 5 invasive SCCs, which were negative for p16, mucosal HPV was not detected.
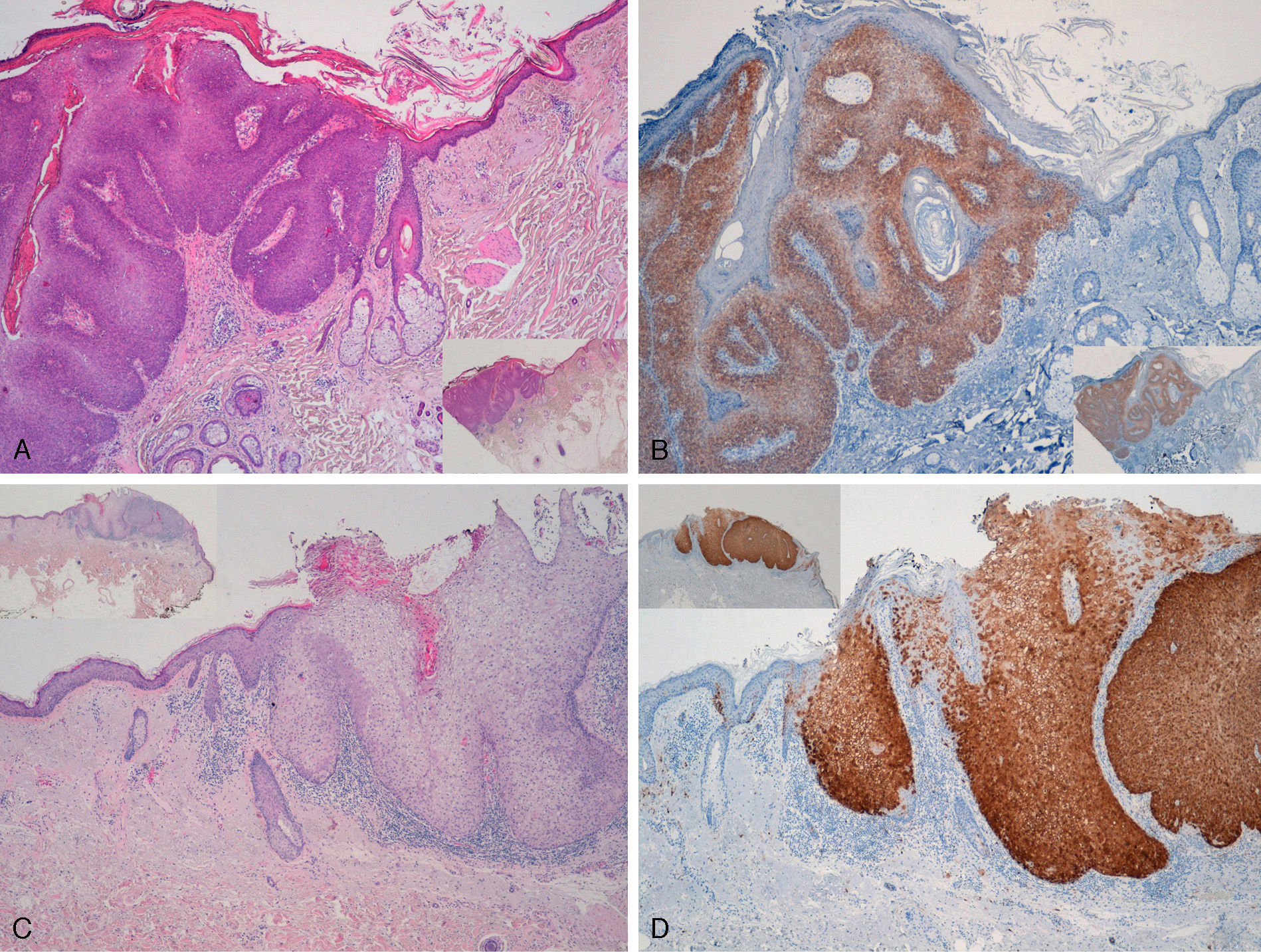
Extragenital LesionsWe observed diffuse positivity in 19 of the 20 intradermal Bowen-disease–type SCCs (positive p16 staining in all epidermal layers affected by the intraepidermal SCC) (Fig. 4); positivity was similar throughout the intraepidermal SCC lesion, regardless of the degree of dysplasia.
Intensely positive staining for p16 in samples from 2 patients with extragenital Bowen disease. A and C, Hematoxylin and eosin staining (original magnification x40). B and D, Staining for p16 (original magnification x40). Insets in C and D show lower-magnification views (original magnification x16).
We detected high-risk mucosal HPV in 4 cases of Bowen disease—HPV-16 in 2 cases and mixed infection with HPV-16 and HPV-11 in 1 case and with HPV-35 and HPV-11 in the other. These were all positive for p16. Three of these 4 Bowen-disease–type intraepidermal SCCs associated with HPV were located on the head and neck and the other was located on the leg.
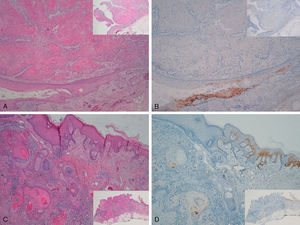
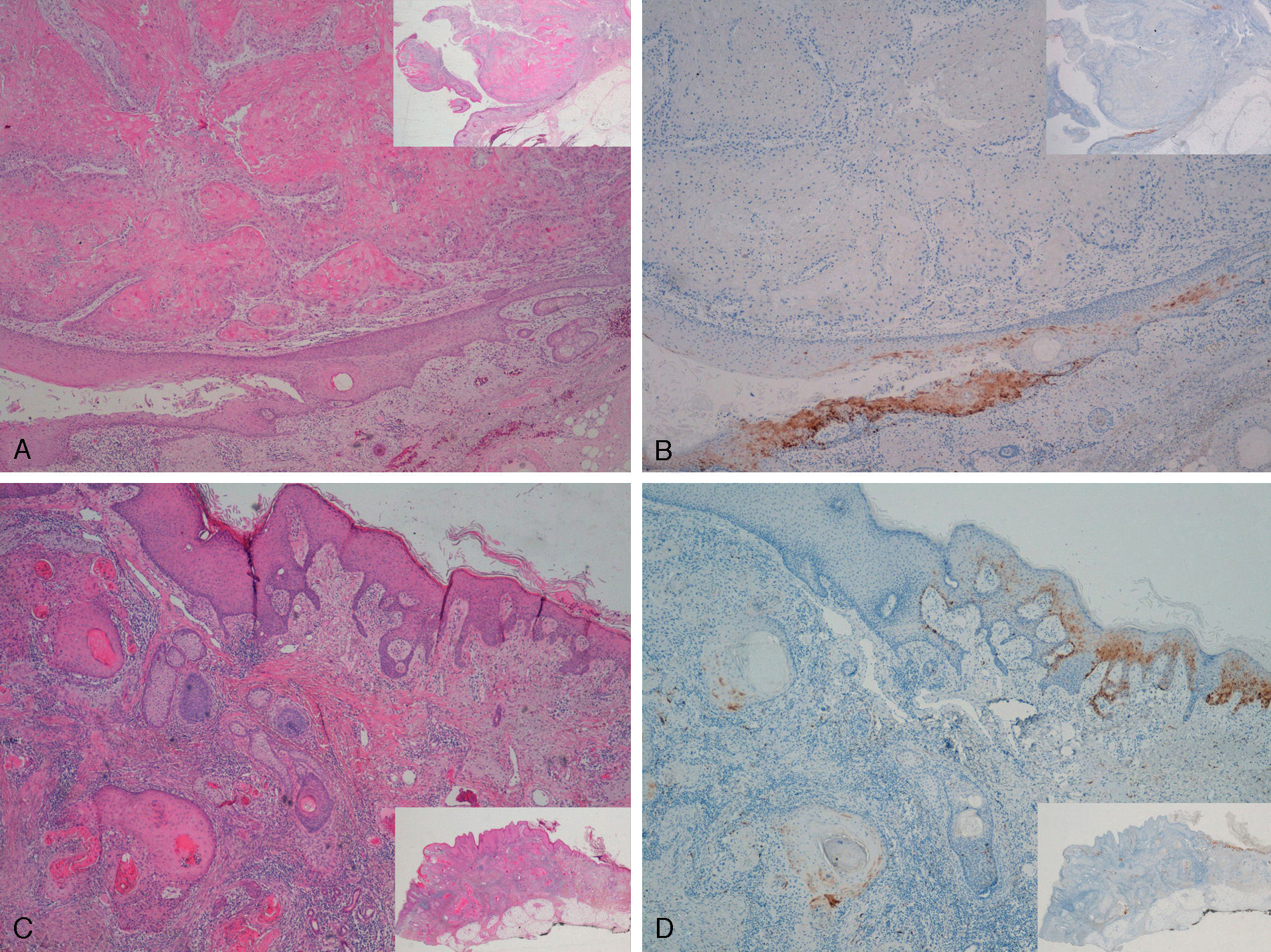
Staining for p16 was negative in 8 of the 10 invasive SCCs; this negativity was observed in the regions where these 8 SCCs were invasive, whereas in the epidermis adjacent to the carcinomas, focal positivity was observed (these were sun-damaged areas with severe dysplasia and/or intradermal actinic-keratosis-type SCC lesions) (Fig. 5). We detected only 1 instance of mucosal HPV—HPV-16—in a p16-immunonegative sample from the outer ear.
Invasive squamous cell carcinoma of the extragenital area. Focally positive staining for p16 in the epidermis adjacent to the carcinoma with severe dysplasia and/or actinic keratosis, negative in the invasive area of the tumor. A and C, Hematoxylin and eosin staining (original magnification x40). B and D, p16 staining (original magnification x40). Insets show lower-magnification views (original magnification x16).
In the statistical analysis, we did not find any relationship between positivity or negativity for p16 staining and mucosal HPV in extragenital lesions in either Bowen-disease–type intraepidermal SCCs or invasive SCCs. In the genital area, there was a relationship between p16 positivity in SCC and mucosal HPV (Fisher exact test, P=.008).
On the other hand, p16 staining was positive in all intraepidermal SCCs, whether genital or extragenital, and negative in invasive SCCs (P<.0001). When analyzed according to genital and extragenital SCCs, the significant relationship only remained for extragenital SCCs (P<.0001).
DiscussionIn SCCs of the cervix and genital region, positive staining for p16 is associated with oncogenic HPV strains (mucosal or α types).6–9 However, this relationship with mucosal HPV is not so clear in extragenital SCCs (particularly Bowen disease–type intraepidermal SCC12–15). In our study, in the case of extragenital lesions, we did not find any relationship between mucosal HPV and p16 staining as p16 status was not associated with presence of mucosal HPV. In the extragenital region, p16 staining was positive in most Bowen-disease–type intraepidermal SCCs—19 of the 20 cases of Bowen disease in our study, in agreement with previous studies of p16 in Bowen disease.12–15 We found that high-risk mucosal HPV was present in 4 Bowen-disease–type SCCs (20%) and all were positive for p16 staining. However, p16 staining was positive in almost all Bowen-disease–type SCCs, regardless of whether mucosal HPV was detected or not. Willman et al14 obtained similar results, and concluded that p16 staining was positive in most Bowen-disease–type intraepidermal SCCs, regardless of the presence of HPV (although they only found mucosal HPV in 3 of the 20 cases [15%] analyzed14). Some studies dating from the late 1990s detected mucosal HPV in a high percentage of extragenital Bowen-disease–type SCCs: Clavel et al20 detected mucosal HPV in 78 out of 94 Bowen-disease–type SCCs (83%), while other authors found similar percentages of mucosal HPV in Bowen-disease–type SCCs of the hands.21,22 In contrast, other studies of Bowen disease did not detect mucosal HPV or did so in much lower percentages, around 15% to 30%.11,23–25 There are a number of explanations for the variability in the results: the HPV types studied, the different PCR and in situ hybridization techniques used, the lesion site (mucosal HPV in hand lesions could be due to self-infection), sample type (fresh, frozen, paraffin embedded [the HPV signal in PCR might not be as strong in paraffin-embedded samples because the DNA might have degraded]), and false positives.11 It could also be that HPV induces oncogenic changes up to a “point of no return” and then disappears or is eliminated by the immune system, rendering it undectable.26,27 Finally, it is possible that there is only a relationship between mucosal HPV and extragenital Bowen-disease–type SCC in certain isolated cases.
On the other hand, in the extragenital area, positivity for p16 in Bowen disease lesions might not be due to the effect of mucosal HPV but rather to the effect of UV radiation or alterations in the cell cycle.5,14,17
In our study, we were able to detect highly oncogenic mucosal HPV such as HPV-16 in some Bowen-disease–type SCCs and in extragenital invasive SCCs, although they were isolated lesions (4 Bowen-disease–type SCCs and 1 invasive SCC; 4 of these 5 lesions were located on the head and neck and the other on the leg). Epidermodysplasia-verruciformis-associated or β HPV has been linked with the appearance of SCCs on extragenital skin. However, these β HPV types do not act on the RB1 gene-p16 protein, as the E7 protein has a very low affinity for RB1, and its oncogenic mechanisms are different to those of mucosal HPV.11 It may be that mucosal HPV is present in some extragenital SCCs in our series, as the PCR technique used only detects mucosal HPV.
From a practical point of view, as p16 staining is diffusely positive in most intraepidermal SCCs, it could be a useful tool for their study, both in the genital and extragenital area. In positive samples, p16 staining can help to clearly delimit these lesions, as well as help determine whether there is any invasion of normal adnexal structures or whether microinfiltration has occurred in the dermis. (In the extragenital area, although sun-damaged skin around the lesion can also be positive for p16, positivity would be focal, occurring in isolated cells, whereas in Bowen disease, diffuse positivity is observed in all layers affected by the disease).
In extragenital invasive SCC, p16 staining is negative in the area invaded by the carcinoma (negative in 8 of the 10 extragenital invasive SCCs) and focally positive in the epidermis adjacent to the SCC (sun-damaged areas with severe dysplasia and/or actinic keratosis lesions). This loss of p16 protein in invasive SCCs compared to intraepidermal SCCs or the epidermis adjacent to the tumor might indicate tumor progression or progression towards an invasive phase, in line with the conclusions of previous studies3,16 and as occurs in some melanomas.28
There were no differences between the findings of our study, performed in Spanish patients, and those of other previous studies performed in other countries.
With regard to the findings for SCC of the genital area, in our study, unsurprisingly, most invasive SCCs of the vulva or penis were negative for p16 staining. It is almost certain that SCCs of the cervix originate via a single oncogenic pathway, associated with mucosal HPV, and both intraepithelial SCCs and invasive SCCs are associated with oncogenic mucosal HPV and these would be p16 positive.9,29 However, in lesions of the vulva and the penis, there may be other oncogenic pathways not related to mucosal HPV. In lesions of the vulva, highly oncogenic mucosal HPV—mainly HPV-16, observed in 75%-80% of intraepithelial SCCs—is detected; these are multifocal lesions in young patients, are mainly associated with the verrucous and basaloid histology, and do not usually progress to invasive SCC. The remaining 20% to 25% are usually unifocal lesions in older patients, associated with differentiated histology. These lesions progress more frequently to invasive SCCs and mucosal HPV types are not implicated in their pathogenesis. Most invasive SCCs of the vulva are derived from these differentiated intraepithelial SCCs of the vulva, and oncogenic mucosal HPV types are only detected in 20% of these invasive SCCs.29 p16 staining is positive in those SCCs associated with oncogenic mucosal HPV types (mostly intraepithelial SCCs) and negative in SCCs of the vulva in which there is no association with mucosal HPV types (for the most part invasive SCCs).9 This would explain our results: although we have studied very few cases, of the 7 invasive SCCs only 2 were positive for p16; we detected high-grade mucosal HPV in the 2 cases positive for p16 but not in the 5 cases negative for p16. The 3 intraepidermal SCCs were positive for p16 staining and we detected mucosal HPV in 2 of them (the other could not be studied because the DNA was degraded).
In genital warts, p16 staining is usually negative,30 in agreement with our results (only a few isolated cells were positive in all cases, along with focal positivity in genital warts with a diffusely positive area). The development of SCCs within genital warts was associated with areas of intensely positive p16 immunostaining.30 In one of our cases, p16 staining allowed us to diagnose changes towards severe dysplasia or in situ SCC (an area with more intense and diffuse p16 positivity; see Fig. 2). Two other genital warts had an area positive for p16, but without evident signs of in situ SCC; these areas might have corresponded to preneoplastic changes (biochemical or genetic changes would be detected earlier than cytological or histological changes). That is, p16 staining could be useful for detecting neoplastic changes in genital warts; we should not forget that HPV-6 and HPV-11, implicated in most genital warts and in the samples in our series, have oncogenic potential, albeit low.27
In conclusion, in our study, we did not find an association between p16 positivity and presence of mucosal HPV in extragenital SCCs; in genital SCCs, although there were a limited number of lesions, there was an association between p16 positivity and oncogenic mucosal HPV, in agreement with previous studies.6–9 In our opinion, our study, which analyzed different carcinomas of genital and extragenital skin, provides a good overview of p16 staining.
We detected highly oncogenic mucosal HPV types, such as HPV-16, in some isolated carcinomas, mostly located on the head and neck. On the other hand, we saw how immunohistochemical staining with p16 can be useful in the study of intraepidermal SCCs on both genital and extragenital sites. Although more studies with a larger number of samples would be necessary for confirmation, loss of the p16 protein in extragenital SCCs seems to indicate progression of the tumor to an invasive phase.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Corbalán-Velez R, et al. Tinción inmunohistoquímica p16 en carcinomas epidermoides del área genital y extragenital. Actas Dermosifiliogr.2001;102:439-447.


![A and B, Intraepidermal carcinoma of the penis with positive staining for p16 in the lower third of the epidermis (A, hematoxylin and eosin staining [original magnification x40]; B, p16 staining [original magnification x40]; insets show lower-magnification views [x16]). C, Intraepidermal carcinoma of the vulva, positive for p16 throughout the epidermis, with some points of microinfiltration in the dermis (original magnification x40, inset shows lower-magnification view [x16]). D, Intraepidermal carcinoma of the penis. Positive staining for p16 throughout the epidermis (original magnification x40, inset shows lower-magnification view [x16]). E and F, Invasive squamous cell carcinoma of the vulva, with p16 staining negative in the tumor (original magnification x40 in E and x100 in F; inset in E shows lower-magnification view [x16]). A and B, Intraepidermal carcinoma of the penis with positive staining for p16 in the lower third of the epidermis (A, hematoxylin and eosin staining [original magnification x40]; B, p16 staining [original magnification x40]; insets show lower-magnification views [x16]). C, Intraepidermal carcinoma of the vulva, positive for p16 throughout the epidermis, with some points of microinfiltration in the dermis (original magnification x40, inset shows lower-magnification view [x16]). D, Intraepidermal carcinoma of the penis. Positive staining for p16 throughout the epidermis (original magnification x40, inset shows lower-magnification view [x16]). E and F, Invasive squamous cell carcinoma of the vulva, with p16 staining negative in the tumor (original magnification x40 in E and x100 in F; inset in E shows lower-magnification view [x16]).](https://static.elsevier.es/multimedia/15782190/0000010200000006/v1_201304241225/S1578219011000151/v1_201304241225/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)