Amputation is the conventional treatment for malignant subungual tumors (MSUTs), namely, subungual squamous cell carcinoma (SUSCC) and subungual melanoma (SUM). Functional surgery consisting of wide local excision (WLE) of the nail unit can preserve function without modifying prognosis in such cases. We present a series of MSUTs treated with WLE of the nail unit, describe the technique, and review its indications.
Material and methodsRetrospective observational study of MSUTs treated with WLE of the nail unit between 2008 and 2017. The technique consisted of en bloc supraperiosteal excision of the nail unit with a margin of 5mm followed by repair with a full-thickness graft.
ResultsEleven MSUTs were treated in the study period: 7 SUMs (4 in situ; mean thickness, 1.17mm; range, 0-4mm) and 4 SUSCCs (mean thickness, 3.4mm; range, 1.6-6mm). WLE of the nail unit was performed in 9 patients and amputation in 2 patients with invasive SUM. Mean follow-up was 39 months (range, 12-96 months) and no local or regional recurrences were detected. One of the 2 patients who underwent amputation developed metastasis to the brain and died. In our review of the literature, we identified 5 series of patients with SUSCC treated with WLE of the nail unit (105 patients) and 14 series of patients with SUM (243 patients). Based on an analysis of these cases and ours, it would appear that WLE of the nail unit is associated with a very low rate of local recurrence (<7%) and offers better functional and cosmetic outcomes than amputation.
ConclusionsWLE of the nail unit is the treatment of choice for SUSCC without bone involvement and for thin noninvasive SUM (Breslow depth <1mm). It is also feasible in intermediate-thickness SUMs when detailed histologic examination of the margins confirms complete resection. Amputation, by contrast, is the treatment of choice for SUSCCs with bone involvement, very thick SUMs (>4mm), and recurrent tumors.
El tratamiento clásico de los tumores malignos subungueales (TMSU), carcinoma epidermoide (CESU) y melanoma (MSU), es la amputación. La cirugía funcional del aparato ungueal (CFAU) puede preservar la función sin modificar el pronóstico. Presentamos nuestra serie de TMSU manejados con CFAU, describimos la técnica y revisamos sus indicaciones.
Material y métodosEstudio observacional retrospectivo de TMSU tratados con CFAU entre 2008 y 2017, con exéresis supraperióstica en bloque del aparato ungueal, margen a 5mm, y cierre con injerto de piel total.
ResultadosSe trataron 11 TMSU, de los cuales 7 fueron MSU (4 in situ, espesor medio: 1,17mm; rango: 0-4mm) y 4 CESU (espesor medio: 3,4mm; rango: 1,6-6mm). Se realizó CFAU en 9 casos y 2 amputaciones en sendos MSU invasivos. El seguimiento medio fue 39 meses, con un rango de 12-96 meses. No hubo recidivas locales ni regionales. Solo un caso —una de las 2 amputaciones—tuvo metástasis (cerebrales) y muerte.
La revisión de la literatura de CFAU en TMSU mostró 5 series (103 pacientes en total) con CESU y 14 series (243 pacientes en total) con MSU. El análisis de nuestros casos y de los casos publicados muestra muy escasas recurrencias locales (<7%), y mejores resultados funcionales y estéticos frente a la amputación.
ConclusionesLa CFAU es de elección en CESU sin afectación ósea y MSU no invasivo o delgado (Breslow <1mm). Es factible en MSU de grosores intermedios siempre con detallado estudio histológico de márgenes que asegure una resección completa. Por el contrario, en CESU con afectación ósea, MSU muy grueso (>4mm) o recurrencias, la amputación debe ser habitualmente de elección.
Malignant subungual tumors (MSUTs), which largely comprise subungual squamous cell carcinoma (SUSCC) and subungual melanoma (SUM), are uncommon tumors in which early diagnosis and adequate surgical management are essential.1 Traditionally, dermatologists used to refer patients with MSUTs to a traumatologist or plastic surgeon for more or less aggressive amputation.2 While amputation achieves good local control, it leaves a considerable functional and cosmetic defect, particularly in the case of finger lesions. Just over 10 years ago, functional surgery consisting of wide local excision (WLE) of the nail unit emerged as an alternative to amputation.3,4 This technique may offer effective control for noninvasive MSUTs and even for larger tumors without other poor prognostic factors.5 WLE of the nail unit preserves the function of the distal phalanx and, compared with amputation, achieves better cosmetic outcomes6 without modifying prognosis. In this study, we present a series of MSUTs treated with WLE of the nail unit at our hospital over a 10-year period (2008-2017), describe the surgical technique, and review its indications.
Material and MethodsPatientsWe undertook a retrospective observational study of the surgical management of patients diagnosed with MSUTs at the Dermatology Department of Hospital General Universitario de Ciudad Real (HGUCR) in Ciudad Real, Spain over a period of 10 years (July 2008 to June 2017). We analyzed demographic, clinical, and histopathologic variables, disease stage at diagnosis, and outcomes (recurrence, metastasis, and death). A survey was designed to collect information on procedure-related complications not reflected in the patients’ charts, postoperative sequelae, and level of patient satisfaction assessed on a Likert scale. The study was approved by the research committee at the HGUCR and complied with regulations governing the publication of patient data. Consent was obtained from all patients.
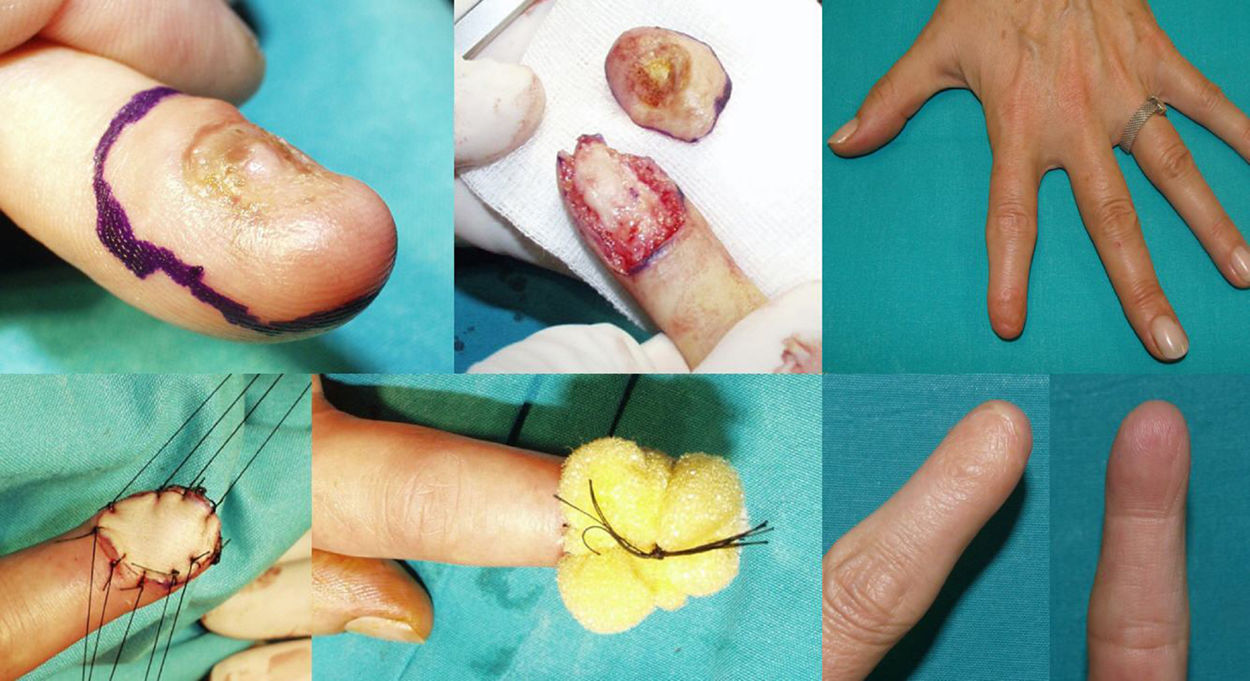
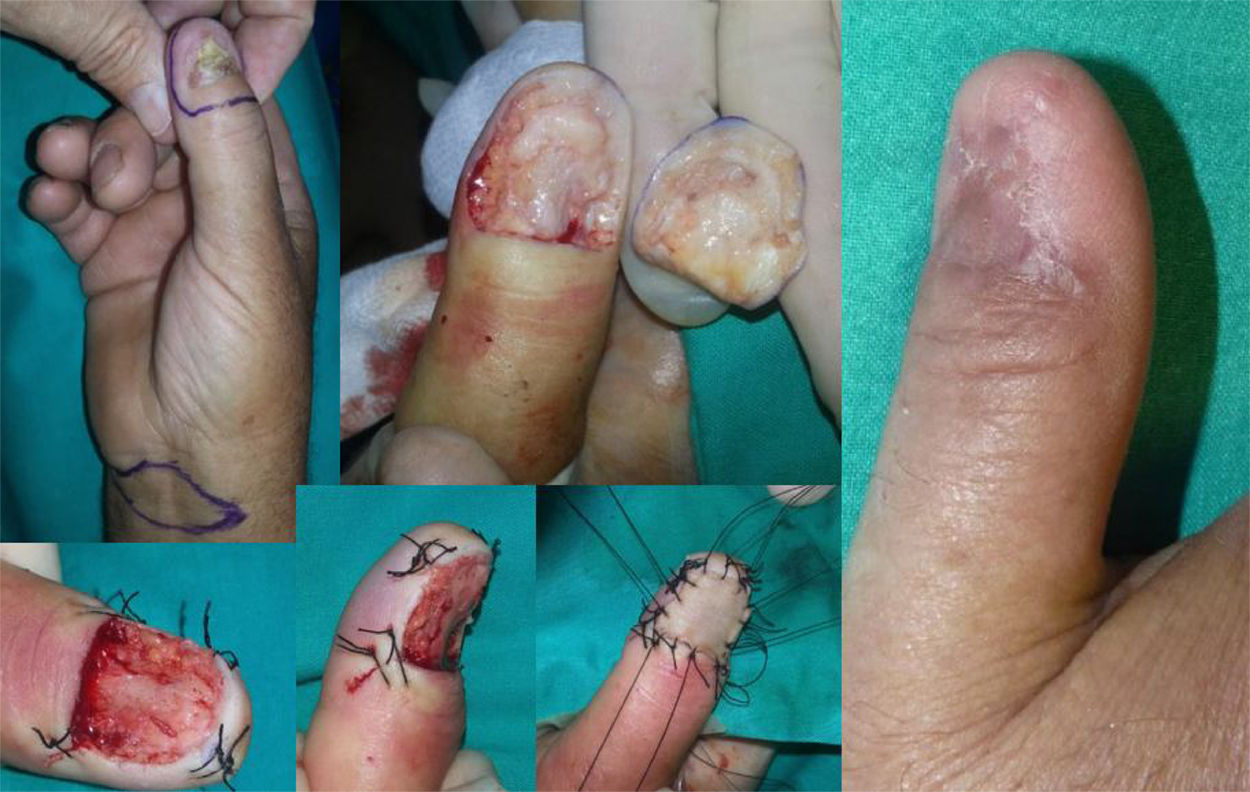
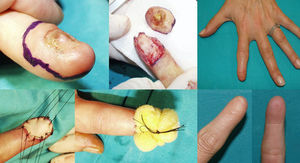
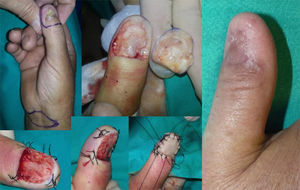
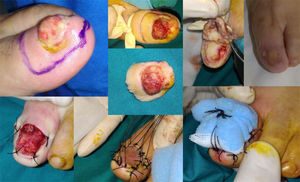
WLE of the Nail Unit: Surgical Technique (Figs. 1-3)En bloc excision of the nail unit was performed using the procedure described below after confirmation of the diagnosis by biopsy.3,7 The procedure is performed under a digital nerve block and hemostasis is achieved with a tourniquet. A margin of 5mm is marked around the nail plate or, when the clinical lesion extends beyond the plate, around the lesion to ensure complete excision. Dermoscopy can help to define the extension of the lesion. Using a #15-blade scalpel, a vertical incision is made down to the periosteum, dissecting the subungual region including the entire nail plate, the lateral and proximal folds, the germinal matrix, and the nail bed. Deep dissection is performed at the level of the underlying periosteum, but the periosteum is spared to prevent damage to the extensor tendon at the point of insertion into the base of the distal phalanx. The excised specimen is oriented appropriately and marked with a silk suture for detailed histopathologic examination. After removal of the tourniquet, the wound is irrigated with normal saline solution and conservative hemostasis is achieved. This procedure leaves a quadrangular defect, which can be reduced in size by dividing the vertical septa, allowing the soft tissue to be advanced circumferentially and dorsally (paronychial flap advancement); additional advancement is made possible by sutures at the 4 corners (Figs. 2 & 3), reducing the size of the surgical defect even further. The rest of the defect is reconstructed using a regeneration template and closed with a full-thickness skin graft (Figs. 1-3).
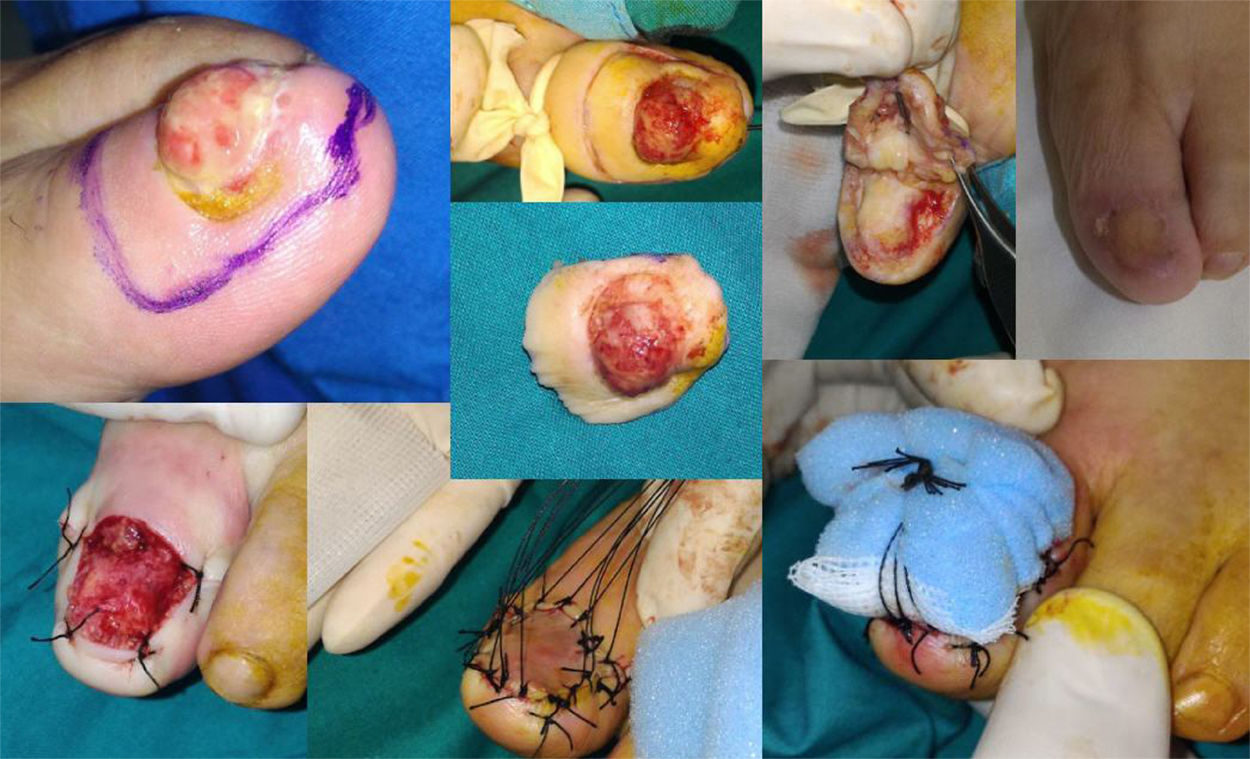
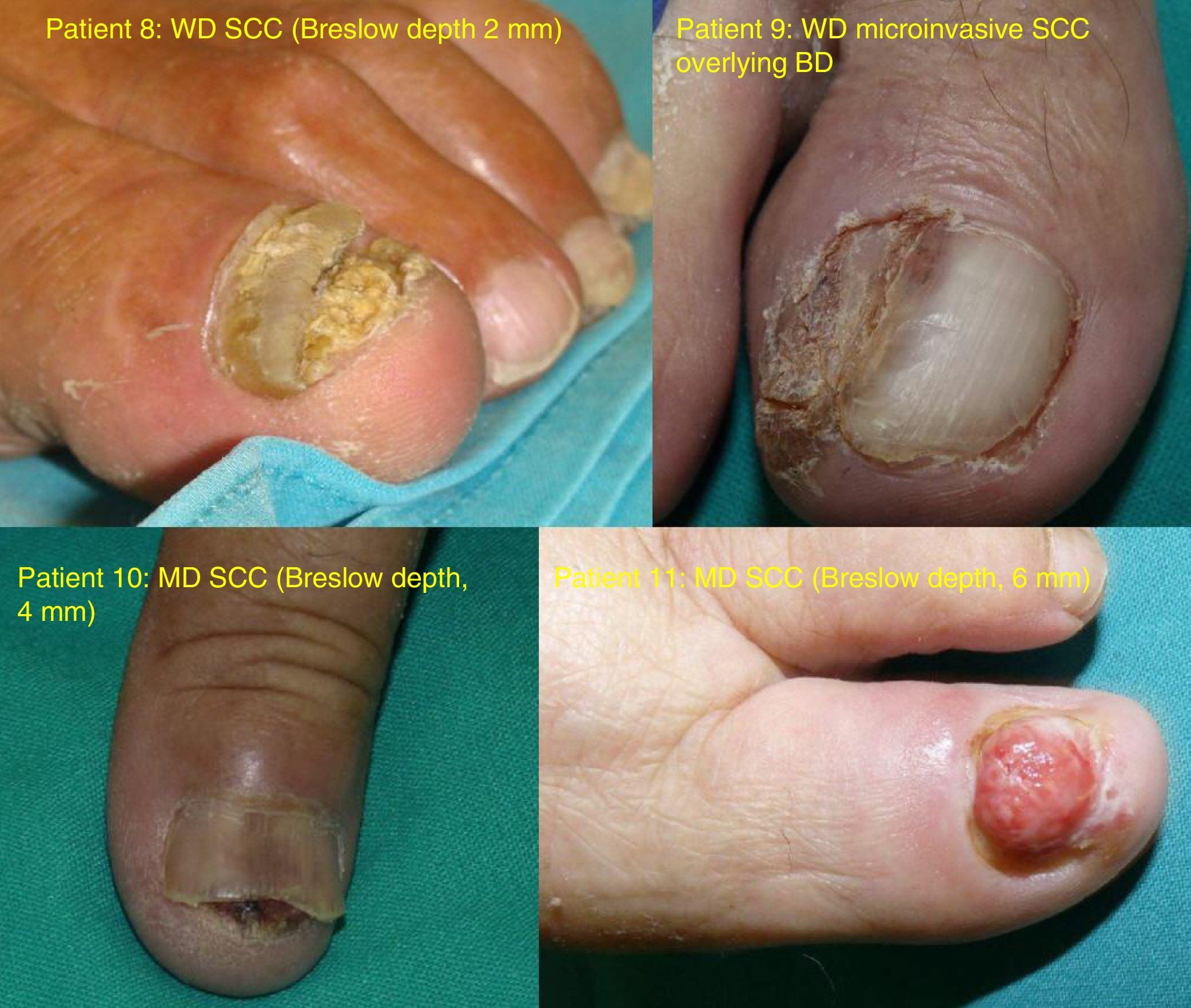
Case 11. Functional surgery with wide local excision of the nail unit in a patient with a moderately differentiated subungual squamous cell carcinoma with a thickness of 6mm on the first toe of the left foot. Note the excrescent tumor in the area of the onychodystrophy. Result after 4 months.
We performed a search in PubMed for articles on the surgical management of MSUTs with function-sparing (functional/wide local) surgery using the following search terms: malignant subungual tumors, subungual melanoma, subungual squamous cell carcinoma, functional surgery, and/or wide local excision in subungual tumors.
The inclusion criteria were original studies containing full evaluations of at least 4 SUMs and/or SUSCCs treated with functional surgery consisting of WLE or variants in addition to information on tumor thickness (Breslow depth) and outcomes (recurrence and mortality) over a minimum follow-up period of 2 years.
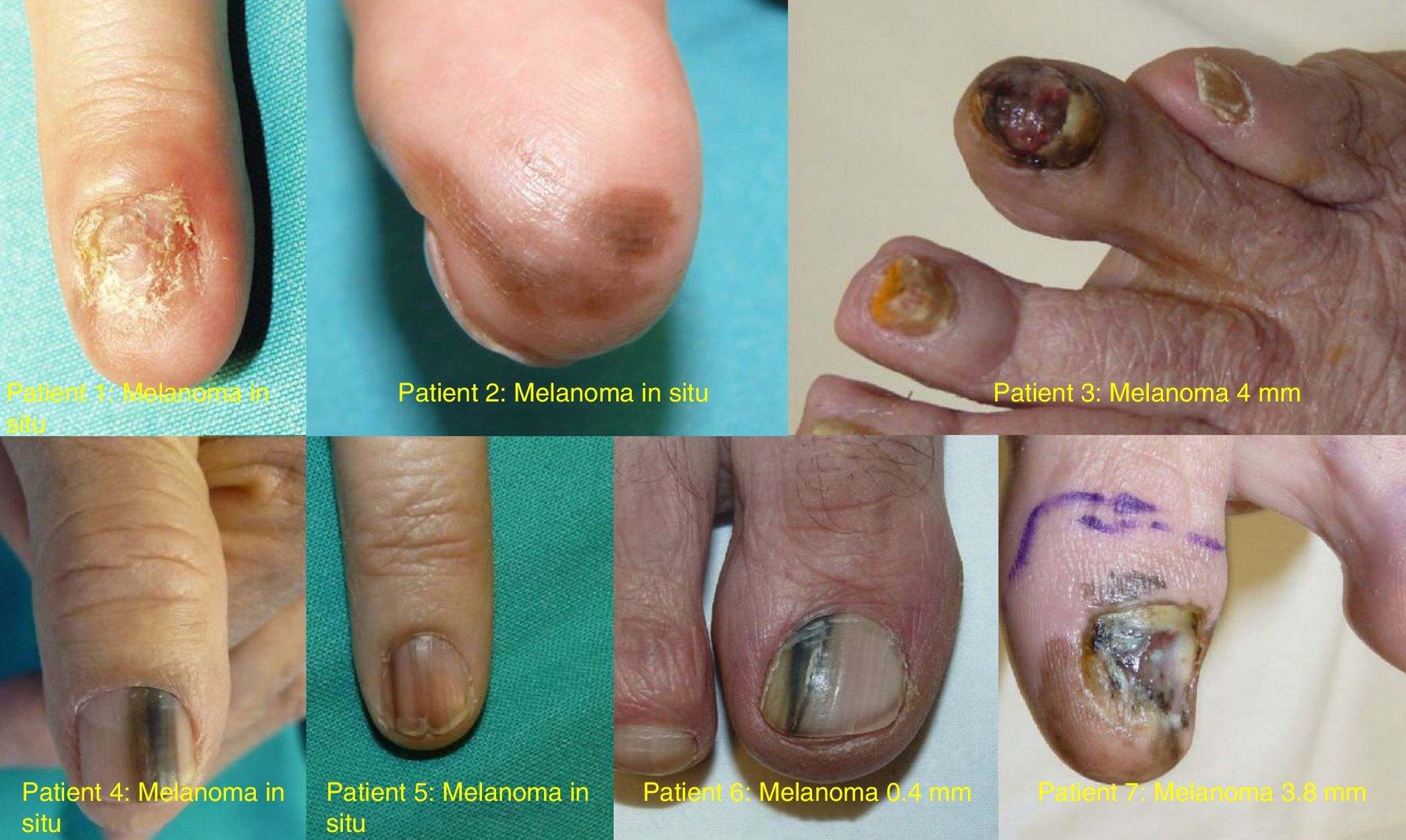
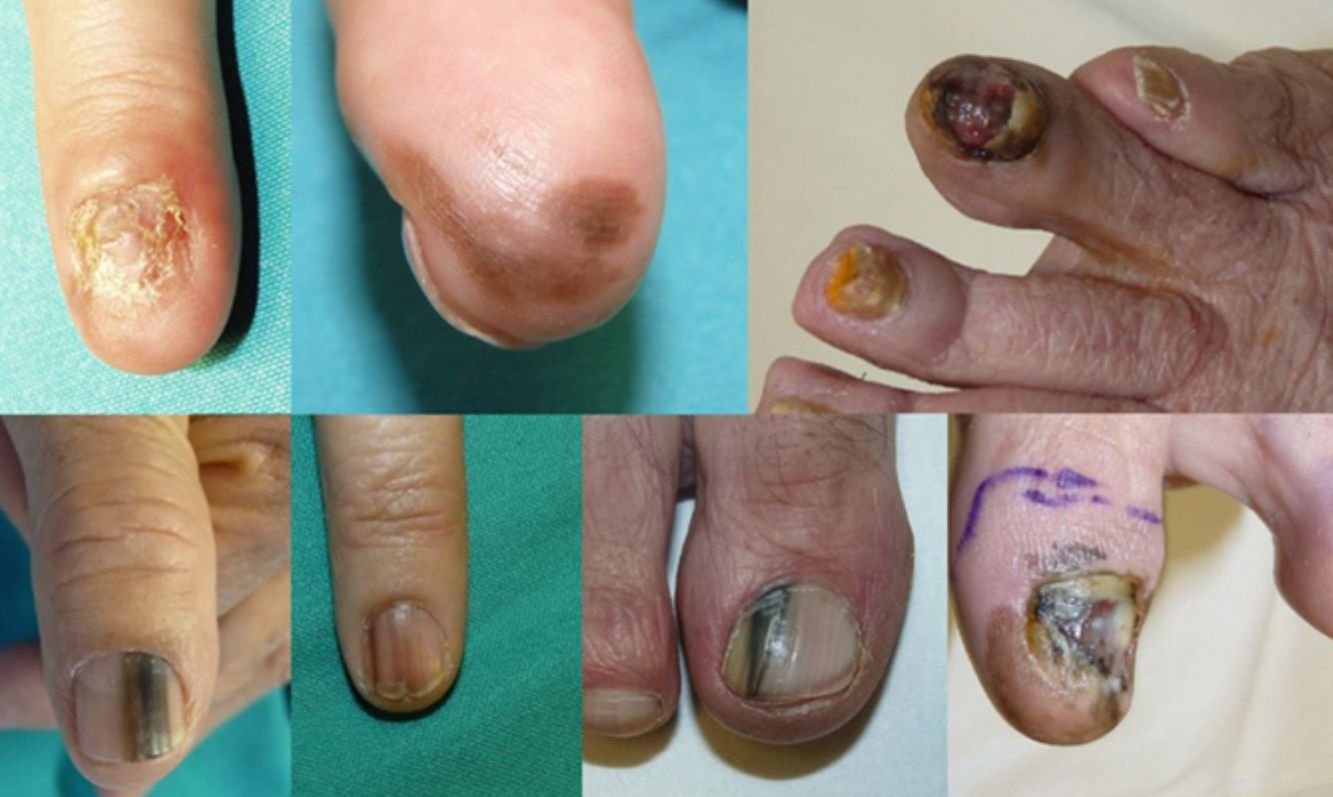
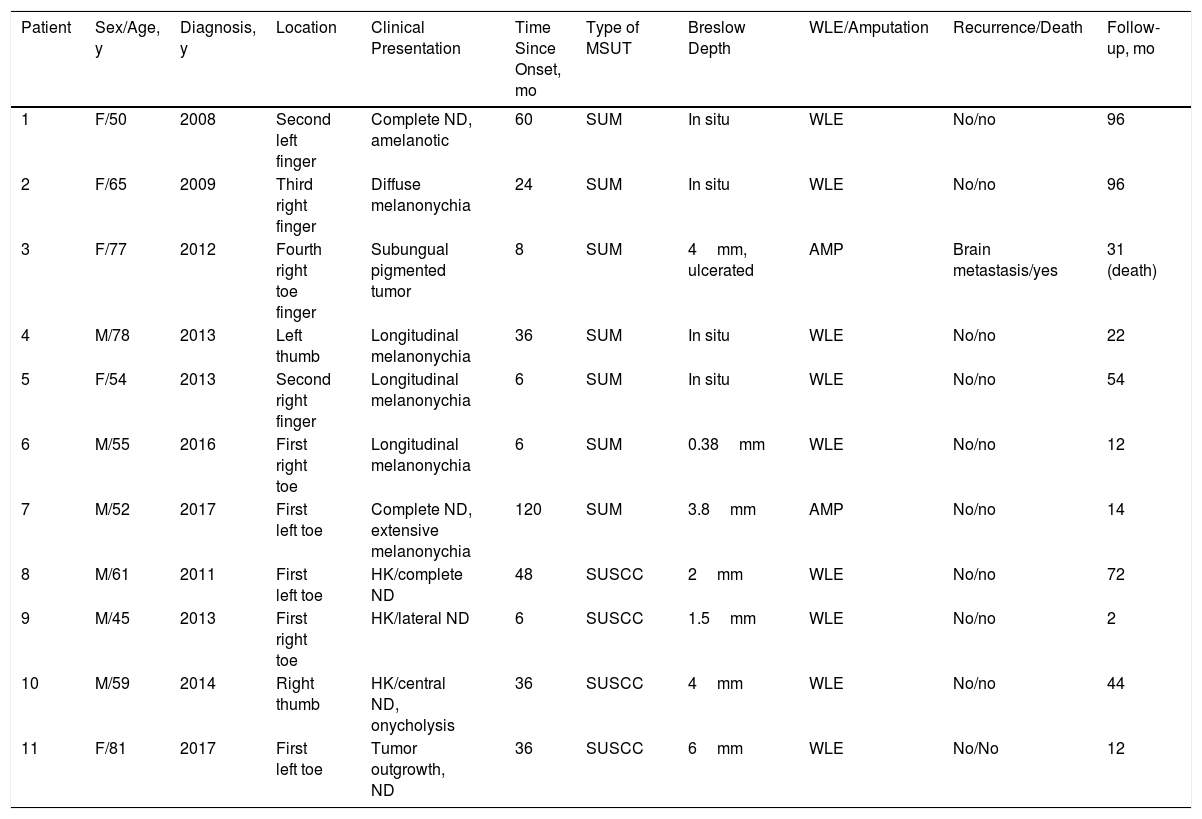
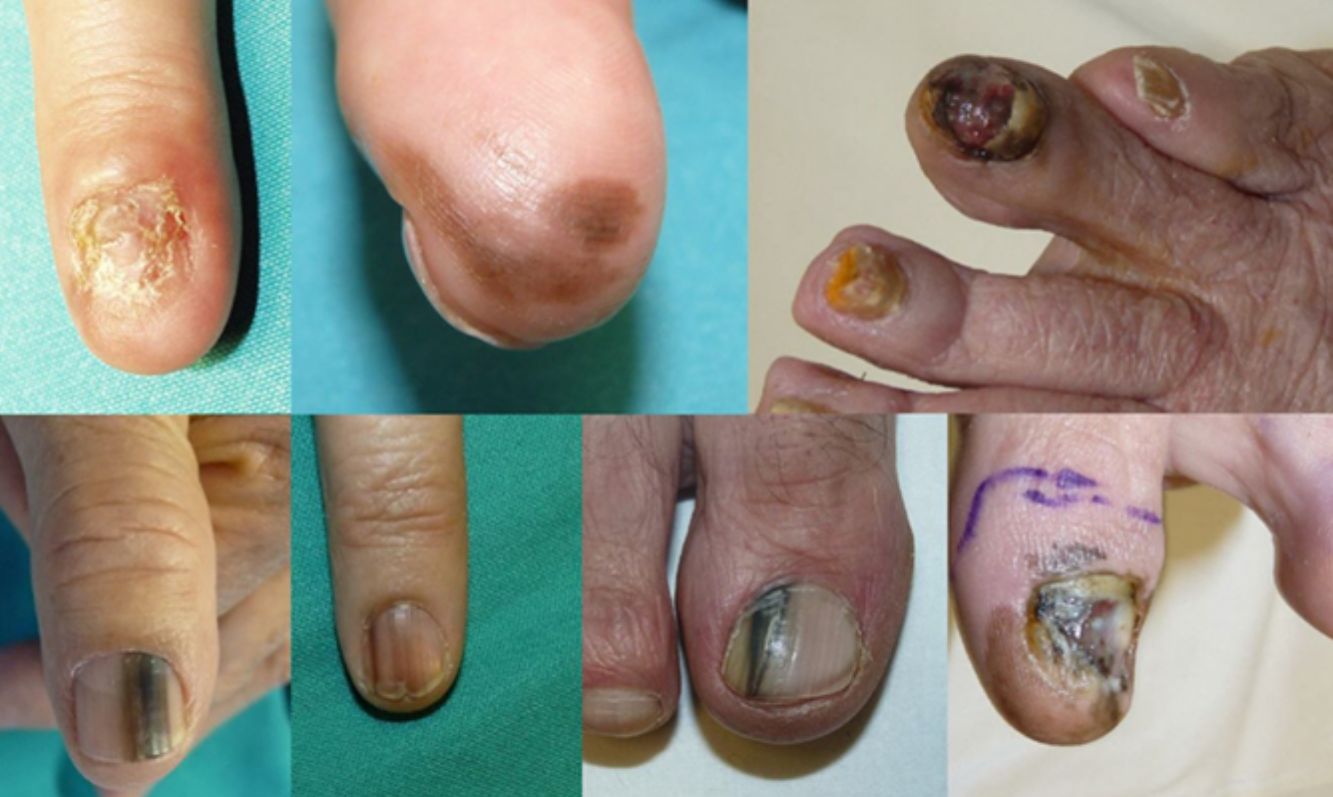
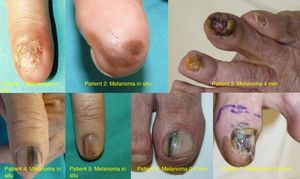
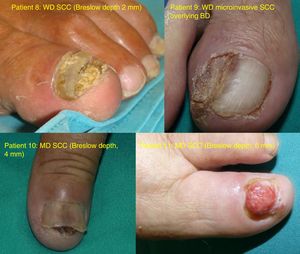
ResultsOver the 10-year period, 11 MSUTs (7 SUMs and 4 SUSCCs) were excised from 5 women and 6 men, all white, aged between 45 and 81 years (mean age, 61 years). The clinical characteristics of the patients (Figs. 4 and 5) and the excised tumors are shown in Table 1. Mean time from onset of symptoms to diagnosis was 35.1 months (range, 6-120 months). This time was similar for SUMs (37.1 months) and SUSCCs (31.5 months). Diagnosis was confirmed by biopsy in all cases, and the 4 patients with SUSCC additionally underwent bone radiography, which was normal in all cases.
Epidemiologic and Clinical Characteristics and Outcomes in Patients With Malignant Subungual Tumors.
| Patient | Sex/Age, y | Diagnosis, y | Location | Clinical Presentation | Time Since Onset, mo | Type of MSUT | Breslow Depth | WLE/Amputation | Recurrence/Death | Follow-up, mo |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/50 | 2008 | Second left finger | Complete ND, amelanotic | 60 | SUM | In situ | WLE | No/no | 96 |
| 2 | F/65 | 2009 | Third right finger | Diffuse melanonychia | 24 | SUM | In situ | WLE | No/no | 96 |
| 3 | F/77 | 2012 | Fourth right toe finger | Subungual pigmented tumor | 8 | SUM | 4mm, ulcerated | AMP | Brain metastasis/yes | 31 (death) |
| 4 | M/78 | 2013 | Left thumb | Longitudinal melanonychia | 36 | SUM | In situ | WLE | No/no | 22 |
| 5 | F/54 | 2013 | Second right finger | Longitudinal melanonychia | 6 | SUM | In situ | WLE | No/no | 54 |
| 6 | M/55 | 2016 | First right toe | Longitudinal melanonychia | 6 | SUM | 0.38mm | WLE | No/no | 12 |
| 7 | M/52 | 2017 | First left toe | Complete ND, extensive melanonychia | 120 | SUM | 3.8mm | AMP | No/no | 14 |
| 8 | M/61 | 2011 | First left toe | HK/complete ND | 48 | SUSCC | 2mm | WLE | No/no | 72 |
| 9 | M/45 | 2013 | First right toe | HK/lateral ND | 6 | SUSCC | 1.5mm | WLE | No/no | 2 |
| 10 | M/59 | 2014 | Right thumb | HK/central ND, onycholysis | 36 | SUSCC | 4mm | WLE | No/no | 44 |
| 11 | F/81 | 2017 | First left toe | Tumor outgrowth, ND | 36 | SUSCC | 6mm | WLE | No/No | 12 |
Abbreviations: AMP, amputation; F, female; HK, hyperkeratosis; M, male; ND, nail dystrophy; SUM, subungual melanoma; SUSCC, subungual squamous cell carcinoma; WLE, wide local excision of nail unit.
Two of the 11 patients had invasive melanoma and underwent amputation. The other 9 underwent WLE of the nail unit with 5-mm margins and deep excision above the periosteum (Figs. 1-3) followed by repair of the surgical defect with a full skin graft, as described in the methods section. Just 1 tumor had surgical margins that needed to be extended after histology showed marginal disease.
The clinical and imaging studies performed after surgery showed local disease in all cases. Polymerase chain reaction detection of human papillomavirus, performed in all patients with SUSCC, yielded 1 positive result.
Mean follow-up time was 39 months (range, 12-96 months). During this period, no local or regional recurrences were observed. One of the patients who had undergone amputation (patient #3) developed brain metastasis and died 31 months after diagnosis of the primary tumor.
Surgical complications included wound infection (3/11), subungual spicules (1/11), moderate stiffness (requiring physiotherapy) that affected the distal interphalangeal joint of a finger (1/11), hypersensitivity to cold (3/11), hypersensitivity to minor trauma (4/11), mild loss of fine-touch sensation (picking up/grasping small objects) in 3 of the 4 patients with finger nail involvement, and reactive hyperkeratosis in 1 patient who had undergone surgery of the first toe. All the complications were mild and transient and improved or practically disappeared over the 1-year follow-up period. Level of patient satisfaction was good and the impact on quality of life was minimal.
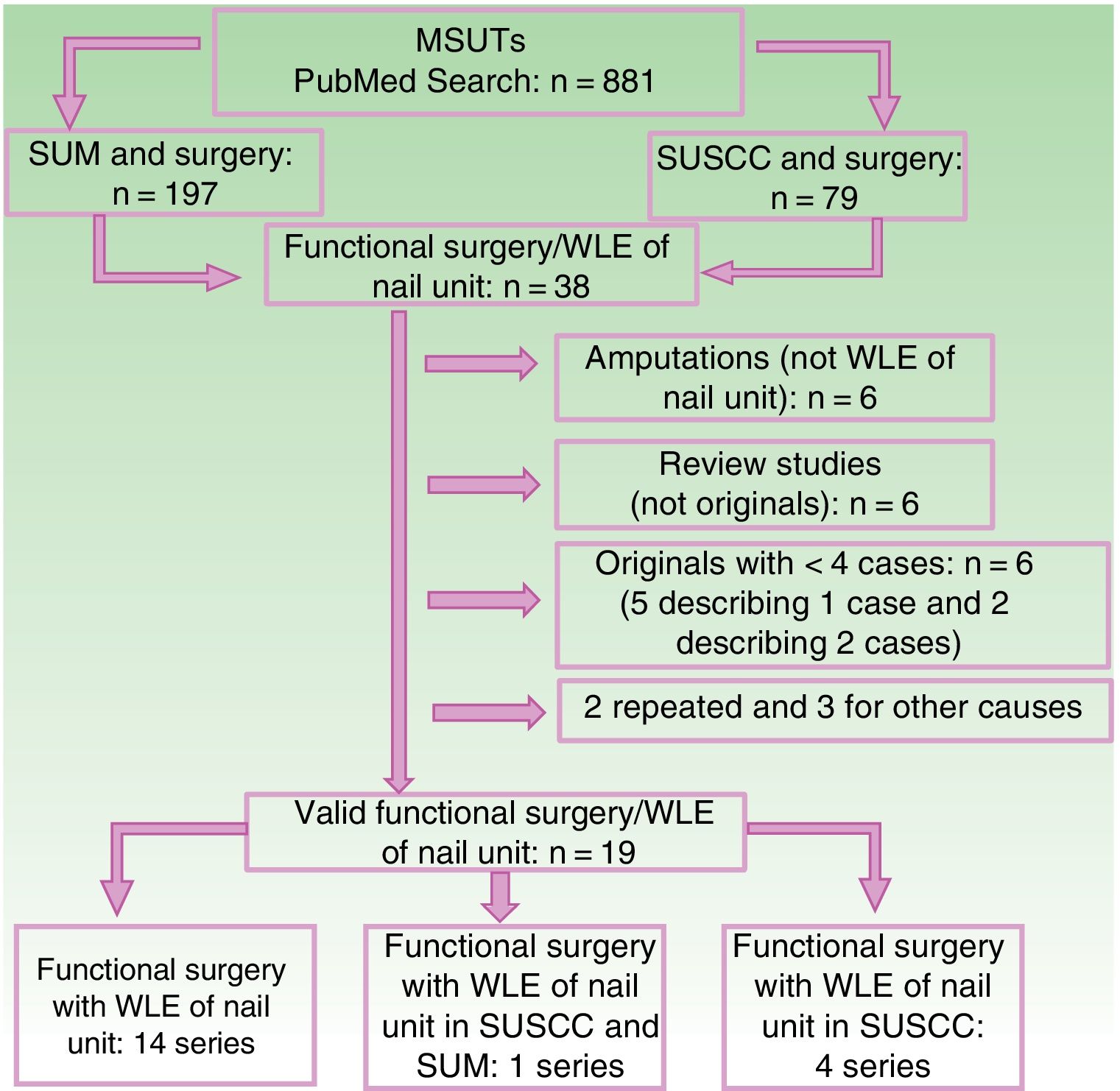
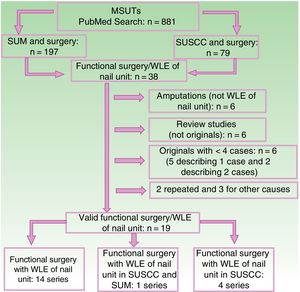
The results of the literature search conducted in PubMed in December 2017 are shown in the flow chart in Figure 6. The resulting evidence for functional surgery with WLE in SUM and SUSSC is summarized in Tables 2 and 3.
Original Series of Subungual Squamous Cell Carcinoma Including Tumors Treated With Functional Surgery Consisting of WLE of the Nail Unit.a
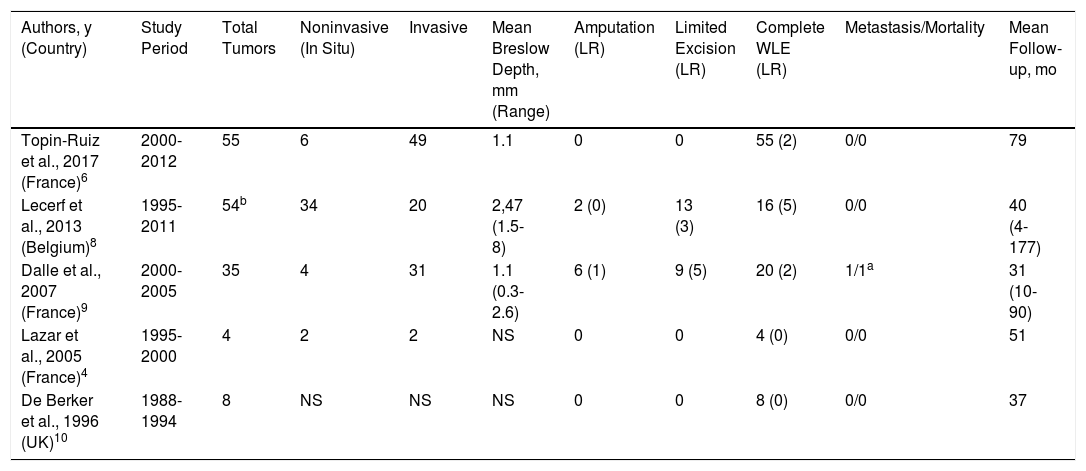
| Authors, y (Country) | Study Period | Total Tumors | Noninvasive (In Situ) | Invasive | Mean Breslow Depth, mm (Range) | Amputation (LR) | Limited Excision (LR) | Complete WLE (LR) | Metastasis/Mortality | Mean Follow-up, mo |
|---|---|---|---|---|---|---|---|---|---|---|
| Topin-Ruiz et al., 2017 (France)6 | 2000-2012 | 55 | 6 | 49 | 1.1 | 0 | 0 | 55 (2) | 0/0 | 79 |
| Lecerf et al., 2013 (Belgium)8 | 1995-2011 | 54b | 34 | 20 | 2,47 (1.5-8) | 2 (0) | 13 (3) | 16 (5) | 0/0 | 40 (4-177) |
| Dalle et al., 2007 (France)9 | 2000-2005 | 35 | 4 | 31 | 1.1 (0.3-2.6) | 6 (1) | 9 (5) | 20 (2) | 1/1a | 31 (10-90) |
| Lazar et al., 2005 (France)4 | 1995-2000 | 4 | 2 | 2 | NS | 0 | 0 | 4 (0) | 0/0 | 51 |
| De Berker et al., 1996 (UK)10 | 1988-1994 | 8 | NS | NS | NS | 0 | 0 | 8 (0) | 0/0 | 37 |
Abbreviations: LR, local recurrence; NS, not specified; WLE, wide local excision of nail unit.
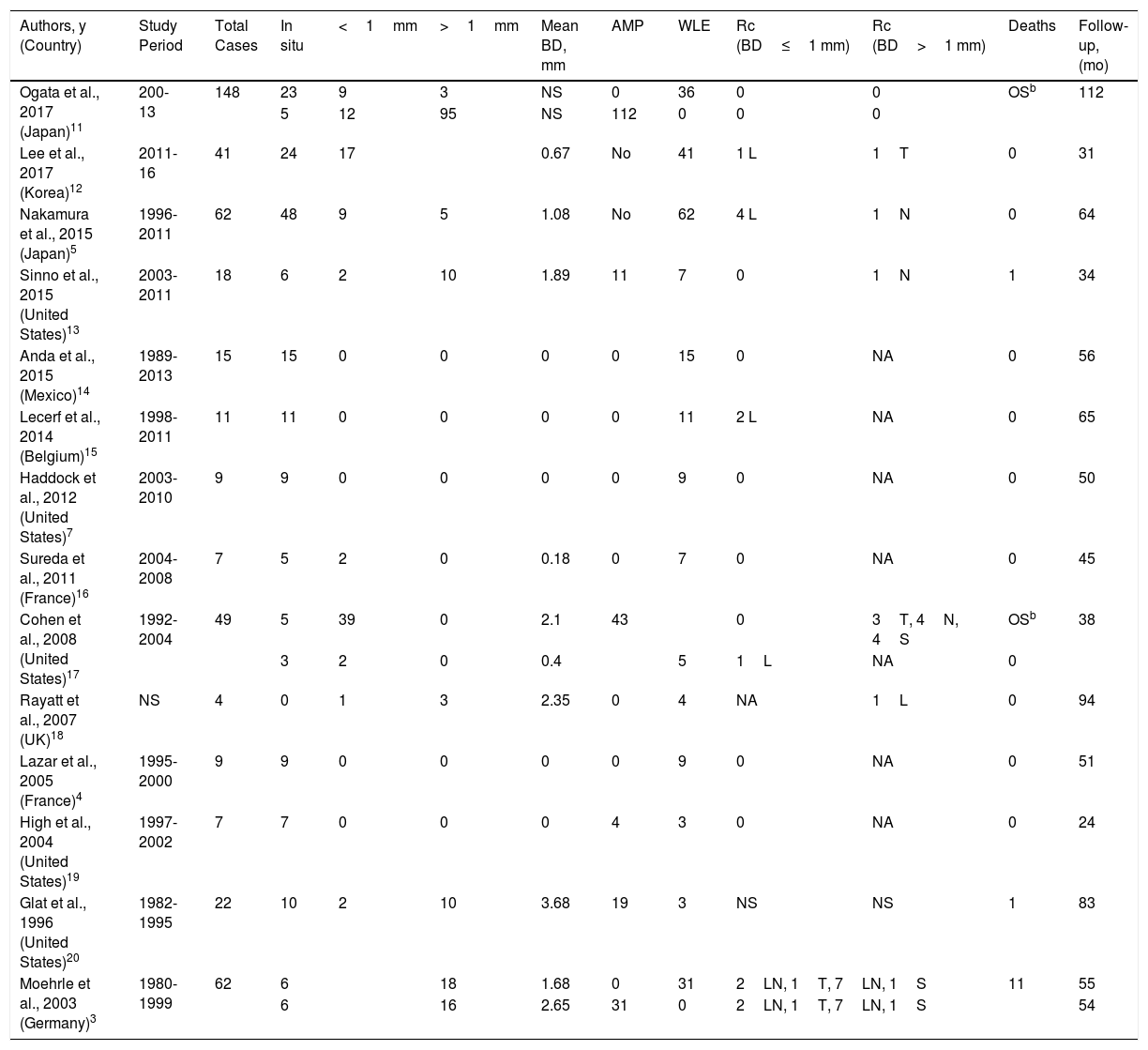
Original Series of Subungual Melanoma Including Tumors Treated With Functional Surgery Consisting of WLE of the Nail Unit.a
| Authors, y (Country) | Study Period | Total Cases | In situ | <1mm | >1mm | Mean BD, mm | AMP | WLE | Rc (BD≤1 mm) | Rc (BD>1 mm) | Deaths | Follow-up, (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ogata et al., 2017 (Japan)11 | 200-13 | 148 | 23 | 9 | 3 | NS | 0 | 36 | 0 | 0 | OSb | 112 |
| 5 | 12 | 95 | NS | 112 | 0 | 0 | 0 | |||||
| Lee et al., 2017 (Korea)12 | 2011-16 | 41 | 24 | 17 | 0.67 | No | 41 | 1 L | 1T | 0 | 31 | |
| Nakamura et al., 2015 (Japan)5 | 1996-2011 | 62 | 48 | 9 | 5 | 1.08 | No | 62 | 4 L | 1N | 0 | 64 |
| Sinno et al., 2015 (United States)13 | 2003-2011 | 18 | 6 | 2 | 10 | 1.89 | 11 | 7 | 0 | 1N | 1 | 34 |
| Anda et al., 2015 (Mexico)14 | 1989-2013 | 15 | 15 | 0 | 0 | 0 | 0 | 15 | 0 | NA | 0 | 56 |
| Lecerf et al., 2014 (Belgium)15 | 1998-2011 | 11 | 11 | 0 | 0 | 0 | 0 | 11 | 2 L | NA | 0 | 65 |
| Haddock et al., 2012 (United States)7 | 2003-2010 | 9 | 9 | 0 | 0 | 0 | 0 | 9 | 0 | NA | 0 | 50 |
| Sureda et al., 2011 (France)16 | 2004-2008 | 7 | 5 | 2 | 0 | 0.18 | 0 | 7 | 0 | NA | 0 | 45 |
| Cohen et al., 2008 (United States)17 | 1992-2004 | 49 | 5 | 39 | 0 | 2.1 | 43 | 0 | 3T, 4N, 4S | OSb | 38 | |
| 3 | 2 | 0 | 0.4 | 5 | 1L | NA | 0 | |||||
| Rayatt et al., 2007 (UK)18 | NS | 4 | 0 | 1 | 3 | 2.35 | 0 | 4 | NA | 1L | 0 | 94 |
| Lazar et al., 2005 (France)4 | 1995-2000 | 9 | 9 | 0 | 0 | 0 | 0 | 9 | 0 | NA | 0 | 51 |
| High et al., 2004 (United States)19 | 1997-2002 | 7 | 7 | 0 | 0 | 0 | 4 | 3 | 0 | NA | 0 | 24 |
| Glat et al., 1996 (United States)20 | 1982-1995 | 22 | 10 | 2 | 10 | 3.68 | 19 | 3 | NS | NS | 1 | 83 |
| Moehrle et al., 2003 (Germany)3 | 1980-1999 | 62 | 6 | 18 | 1.68 | 0 | 31 | 2LN, 1T, 7LN, 1S | 11 | 55 | ||
| 6 | 16 | 2.65 | 31 | 0 | 2LN, 1T, 7LN, 1S | 54 | ||||||
Abbreviations: AMP, amputation; BD, Breslow depth; L, local; N, nodal; NS, not specified; OS, overall survival; Rc, recurrence; S, systemic; T, transit; WLE, functional surgery with wide local excision of nail unit.
Our findings (Table 1) and the results of the literature review (Tables 2 and 3) indicate that functional surgery consisting of WLE of the nail unit is a good alternative to amputation in MSUTs, as it is associated with low recurrence (<7%) and better functional and cosmetic results. WLE is associated with high patient satisfaction, good local disease control, and no differences in survival.
The nail unit is a complex structure that can be affected by a wide range of benign and malignant tumors.1 MSUTs are uncommon and mainly consist of SUSCC and SUM. Both of these entities have variable clinical presentations and require a high level of suspicion to prevent diagnostic delays, which can complicate treatment and negatively affect prognosis.6,7 Because MSUTs are rare, there is little evidence on the best treatment strategies, and exhaustive literature reviews are thus necessary to identify the best available evidence.21
SCC is the most common MSUT. SUSCC affects middle-aged men and is more common on the hands.6,9,22 It can mimic benign conditions, causing diagnostic delays. Similar conditions include onychomycosis, nail dystrophy induced by trauma or inflammatory disease, exostosis, verruca vulgaris, pyogenic granuloma, and chronic paronychia.6,23 Metastasis and mortality are rare in SUSCC.6
The second most common MSUT is melanoma. SUM is rare in whites, in whom it accounts for just 2% to 3% of all melanomas.24,25 It is common, however, in patients with high Fitzpatrick skin types. In blacks and Asians, for example, it represents 20% of all melanomas.5,11 It affects both men and women starting in the fifth decade of life, and is more common in the first fingers and toes. Early presentations include irregular pigmented bands; later manifestations include the Hutchinson sign and, once advanced disease has set in, dystrophic nail changes. Lesions may also be amelanotic, however, resulting in diagnostic delays.25,26
Subungual disease is a risk factor in malignant melanoma, but it is not clear whether this is because the tumor is diagnosed later11,25 or because it is biologically more aggressive.24 Nail keratin increases the dispersal of light in melanocytic lesions, causing what is known as the Tyndall effect,21 and this can makes it more difficult to classify lesions. Mean time to diagnosis is very long in these patients. In our series, the mean time from the onset of symptoms to diagnosis was 35 months. Mean Breslow depth was 1.16mm; 4 of the 7 tumors (57%) were in situ but 2 (28%) had a Breslow depth of over 3.8mm. Similar data have been observed in other studies, which have reported prevalence rates of 18% to 25% for SUM in situ,11,24 albeit with a high rate of thick melanomas (29%-50% measuring >4mm).11,24,25 Factors linked to high mortality in SUM are similar to those seen in malignant melanoma in nonacral locations: Breslow depth, ulceration, a positive sentinel lymph node biopsy, and tumor stage at diagnosis.11,24 Diagnostic difficulties and slow growth are common in acral lentiginous melanoma and Bowen disease and in well-differentiated SUSCC. High mortality rates (30%-40% at 5 years) have been reported in some series of SUM.2,22,26 By comparison, SUSCC exhibits particularly benign behavior. In the 2 largest series of SUSCC to date, comprising 104 patients, there were no cases of metastasis or death.6,8 Based on data in the literature up to 2013, there have been just 10 cases of metastasis and 3 deaths due to SUSCCC.8
There are no clear recommendations on the surgical management of MSUTs.6,13 SUM is not mentioned in the European Society for Medical Oncology27 or the Scottish Intercollegiate Guidelines Network guidelines.28 While it is mentioned in the American Academy of Dermatology (2011)29 and National Comprehensive Cancer Network30 guidelines, the recommendations are limited to preoperative biopsy. The only guidelines (currently being revised) that offer guidance on the management of SUM are the Australian ones (2008).31 These recommend adjusting the surgical margins to Breslow depth where possible, and mention the possibility of partial amputation at the level of the proximal joint located closest to the melanoma. The guidelines, published in 2008, state that “functional” amputation may be considered for subungual melanoma, as the little evidence available does not show advantages for radical amputation.
The general lack of recommendations on the surgical management of MSUTs contrasts with reports over the last decade of more conservative techniques than conventional amputation being used to treat MSUTs (Table 3). The effectiveness of the different surgical modalities13,25 and their influence on overall survival,5,6,17 however, are still a topic of debate, partly because of the absence of large series and the shortage of multicenter studies. Classic amputation, which can vary in terms of its completeness,2,26 can cause functional problems, poor cosmetic outcomes, and even psychological problems.32 Amputation of the thumb has been associated with a 10% loss of function when performed at the interphalangeal joint but a 40% loss when performed at the metacarpophalangeal joint5,7,32 due to the shortening and deinsertion of the distal flexor tendon, which can make it difficult to pick up or grasp small objects.32 In addition, patients may experience loss of sensation in the amputated area, causing interference with certain activities of daily living.
It has been known since the 1990s that more extreme amputation does not offer any benefits in terms of recurrence or survival in SUM.2,33–35 This finding led to the idea that functional surgery consisting of wide local excision with en bloc removal of the nail unit, without bone amputation, could be an effective alternative. In 2004, Moherle et al.3 published a series of 62 SUMs, half of which were treated with amputation and half of which were treated with WLE of the nail unit. They found no differences between the groups for disease-free or overall survival, and there were just 2 local recurrences in the WLE group. Of interest was the use of 3-dimensional histology to check for tumor-free excision.
The results for the series identified in the literature search (Tables 2 and 3) show that 243 SUMs and 103 SUSCCs were treated by functional surgery consisting of WLE of the nail unit. Most of the MSUTs were in situ, thin, or minimally invasive and WLE produced excellent functional and cosmetic outcomes, without compromising prognosis. There were very few recurrences and they were mainly local. The local recurrence rate for SUSCCs was 8.7% and there were no metastases or deaths in the 103 patients analyzed. The local recurrence rate for SUM was 4.9% (12/243). The rate for other types of recurrence was just 4% (2 satellite and 8 lymph node metastases). Most of the tumors were in situ tumors or tumors with a Breslow depth of less than 1mm. Just 30 tumors treated with WLE had a Breslow thickness of over 1mm. The procedure in all cases consisted of en bloc excision of the nail unit for histopathologic examination with recommended surgical margins of 5mm to 10mm. Excision may11 or may not3 include the periosteum and neither option appears to affect surgical or survival outcomes. Circumferential and dorsal advancement of the lateral and distal margins (paronychial advancement flap) reduces the size of the surgical defect, facilitating graft repair. In the earliest reports of WLE of the nail unit, surgical wounds were left to heal by secondary intention,10 but it is now possible to use skin replacement systems (INTEGRA).5 Both options would be favored when the periosteum is excised or when doubts about the involvement of margins arise during surgery. When WLE of the nail unit first emerged, tumor clearance was checked with Mohs micrographic surgery10 or 3-dimensional histology.3 Now, however, it is not clear whether these techniques are useful for nail lesions, particularly in cases of incomplete nail excision, as the anatomic complexity of the folds makes it difficult to interpret findings.6,9 Whatever the case, meticulous histology is required to ensure complete excision.
There is an interesting discussion about the short distance between the nail matrix and the bony ridge, which is just 0.9mm according to 1 cadaveric study.36 Some authors have argued that this short distance is the main limiting factor for WLE versus amputation in the case of invasive (not noninvasive) tumors.5 That said, an anatomic study of 30 amputation specimens from patients with invasive SUM found no bone involvement in 14 SUMs with a Breslow depth shorter than 4mm and bone involvement in just 4 of the 16 SUMs with a Breslow depth of over 4mm.37 The authors concluded that functional surgery with WLE of the nail unit was feasible for intermediate-thickness tumors up to 4mm. Our literature review (summarized in Tables 2 and 3) clearly suggests that functional surgery with WLE is favored over amputation for noninvasive (in situ) or minimally invasive (<1mm) MSUTs.6,16 Its indication for thicker lesions is less clear,5,22 but it has been used for both SUM and SUSCC without other poor prognostic factors and for patients who have refused amputation.38 Local recurrence rates are low in SUSCC (8.7%) and SUM (4.9%). Of the 103 SUSCCs treated with WLE of the nail unit in the literature, there were no cases of regional recurrence or mortality. In the case of SUM, none of the patients with a tumor with a Breslow depth of less than 1mm developed regional metastasis or died. Like other authors,3,5 we believe that the potential for metastasis depends on known risk factors (Breslow depth, ulceration, sentinel lymph node biopsy positivity) and not on the aggressiveness of the local surgery. The surgical approach should be sufficient to ensure complete removal of the tumor. In this regard, en bloc WLE of the nail unit, without amputation, should be an attractive option for a large proportion of patients. Tumors treated with limited resection or tumorectomy have a greater risk of recurrence and subsequent spread if remnant tumor is detected late. Tumorectomy in MSUTs produces margins that are difficult to examine histologically and it can also leave nail remnants with worse cosmetic outcomes than en bloc excision (WLE). In the case of thicker tumors (Breslow depth >1mm), functional surgery consisting of WLE of the nail unit is feasible (although opinions are more divided on this) and carries a lower risk in SUSCC1 than in SUM.8,23 Amputation at the most distal point possible should be reserved for invasive tumors with bone or joint involvement, recurrent lesions, and lesions with a Breslow depth of over 4mm.3,5,6 In the case of SUMs with an intermediate thickness (1-4mm), the risks and benefits of functional surgery with WLE of the nail unit should be discussed with the patient, and if it is decided to go ahead, detailed histologic examination of the margins is essential.3,5,11
Complications of WLE of the nail unit include wound infection, inclusion cysts, nail spicules, joint stiffness, reactive hyperkeratosis, pain and/or digital hypersensitivity to mild trauma, hypersensitivity to cold, and loss of fine-touch sensation.1,2,20 These adverse effects are well tolerated and, as our findings also show, have little or no effect on quality of life.1,20
The limitations of our study include its retrospective design, the small number of cases included, and the analysis of 2 tumors that require similar surgical treatment due to their location but have different origins and biologic behavior. Notable strengths include the detailed individual study of patients and our extensive literature review. Because MSUTs are so uncommon, it would be interesting to perform multicenter studies and/or create registries to compile a sufficient number of cases to better define and standardize indications.
ConclusionsBased on the evidence available for MSUTs, functional surgery consisting of WLE of the nail unit is the treatment of choice for SUSCCs without bone involvement and for noninvasive or thin (Breslow depth <1mm) SUMs. The data from our series and the literature indicate that WLE of the nail unit is associated with low recurrence and offers better functional and cosmetic outcomes than amputation. It can be considered in patients with intermediate-thickness SUMs (1-4mm), but it should always be followed by meticulous histologic examination of the surgical margins to ensure excision completeness. Amputation, by contrast, would appear to be the treatment of choice for SUSCCs with bone involvement, very thick SUMs (>4mm), and recurrent tumors.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Flores-Terry M, Romero-Aguilera G, Mendoza C, Franco M, Cortina P, Garcia-Arpa M, et al. Cirugía funcional en tumores malignos subungueales. Serie de casos y revisión de la literatura. Actas Dermosifiliogr. 2018;109:712–721.